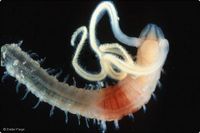
Benthos
Definition of Benthos:
Benthos, also called zoobenthos, refers to benthic organisms that live either just above the bottom but closely associated with it (hyperbenthos), on the sediment surface (epifauna), or among the sediment grains (infauna).[1] Photosynthesizing benthic organisms (cyanobacteria, algae, plants) are often referred to as phytobenthos.
This is the common definition for Benthos, other definitions can be discussed in the article
|
Benthic fauna is an essential component of the marine ecosystem. Benthic organisms make a major contribution to the decomposition of organic matter and the cycling of nutrients in the marine environment. They not only fuel in this way the primary production in the water column but they also constitute themselves an important food source for marine organisms at higher trophic levels. They create ecological niches for themselves and for other benthic species by conditioning the substrate on which they live. They are highly sensitive to changes in environmental conditions and can therefore fulfill a sentinel role.
Contents
Benthic habitats
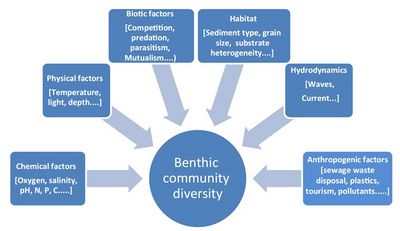
Benthic communities can be distinguished according to the type of habitat they live in. Many benthic animals are so-called eco-engineers, which actively contribute to shaping these habitats (see Biogeomorphology of coastal systems). Some benthic animals destabilize the substrate (burrowing, feeding activities), while others stabilize the substrate (mucus secretion). Common benthic habitats in the marine environment are mangroves, seagrass meadows, kelp forests, coral reefs, rocky shores, sandy shores, soft sediments, and deep-sea benthic habitats.[2] These habitats provide essential environmental conditions to which the benthic communities are adapted. Relative to the pelagic zone, the seafloor presents a greater variety of physically diverse habitats that differ from each other in terms of depth, temperature, light availability, salinity, degree of immersion (tidal vs. subtidal), and type of substrate (Fig. 1). The great diversity of benthic habitats is reflected in the great diversity of benthic animal species. The number of benthic animal species exceeds one million and exceeds by far the number of pelagic animal species.[3]
The dissolved oxygen in combination with other factors such as organic carbon content, sediment size, and water depth strongly influence the abundance and diversity of the benthos assemblage. Oxygen diffusion into the seabed is slow, especially in muddy sediments, and generally limited to the top millimeters in the absence of bioturbation. Polychaetae species (bristle worms) are favored due to their low-oxygen environment tolerance and fast recruitment.[4] Through bioturbation, oxygen can penetrate several centimeters into the seabed. The combination of limited food and oxygen penetration makes that the vast majority of benthic organisms and most of the primary production are confined to the upper few centimeters of sediment near the sediment–water interface.[1]
Coarse-grained sediment beds have lower benthic diversity and population than fine-grained beds. Coarse-grained beds are typical of high-energy hydrodynamic environments, which constitute a less suitable habitat for many benthic organisms. In addition, fine sediments - clay particles in particular - can easily bind with other substances in the water (see Coastal and marine sediments). The content of organic matter is therefore generally much higher in fine-grained substrates than in coarse-grained substrates. The resulting difference in food availability is another factor that favors fine-grained sediment beds over coarse-grained beds as a popular settlement site for benthic organisms.[5] But there are also tradeoffs: fine-sediment beds are less oxygenated and adsorb toxic contaminants more easily.
Classification of benthos
Benthic animals can be categorized according to size. Megafauna are large benthic organisms such as flatfish, scallops, crabs, starfish and gastropods (slugs, snails). Megafauna are typically predators and scavengers. Predators keep grazers from depleting resources and are therefore crucial for maintaining the ecosystem. Macrofauna are smaller organisms that are retained on a 1 mm sieve. This size grouping includes polychaetas, crustaceans, bivalves, and many other phyla. Their abundance in coastal waters ranges from 500 to 10,000 ind/m2, while biomass ranges from 5 to 200 g/m2. For meiofauna, the abundance typically ranges from 105 to 107 ind/m2. [5] Meiofauna refers to organisms that are retained on a 45-µm sieve (the exact dimensions will vary from researcher to researcher) and are usually dominated by nematodes and other dominant groups of copepods, rotifers, tardigrades and ostracods, with densities in the order of one million per square meter. Benthic foraminifera and ostracods are meiofaunal species used as ecological indicators of the depositional history of coastal environments, due to their tiny size, abundance, and specific adaptations to different environmental conditions. Microfauna include living organisms that pass through a 45-µm sieve and include bacteria and protists. They play an important role in returning nitrate and phosphate to the pelagic. Some anaerobes metabolize sulfur and release hydrogen sulfide, ammonia, and other toxic reduced ions.
 Gastropod Homalopoma sangarense. Courtesy Naturalis |
 Rotifer Brachionus manjavacas, microscopic planktonic organism. Courtesy Kristin Gribble. |
 The myodocopid ostracod Vargula hilgendorfii (sea firefly), famous for its bioluminescence. Courtesy R.J. Smith |
 Milnesium tardigradum. Tardigrades (water bears) are among the most resilient animals known, capable to survive extreme conditions. Courtesy E. Schokraie et al. (2012[6]) |
 Foraminifera Anomalinoides Sphericus. Courtesy B.W. Bruce et al. (1999[7]) |
Benthic communities that occur below photosynthetic depths rely on phytoplankton sinking from surface waters above and macrophyte detritus transported by currents from nearshore environments. Most benthic organisms are either deposit feeders or suspension feeders.
Deposit feeders live from detrital sediments (including associated microbes) and benthic algae (diatoms). Deposit feeders are mainly found in quiescent areas, characterized by low waves, small currents and relatively stable fine-grained substrate. Quiescent areas occur in deep water environments (seabed below the penetration depth of waves) or in sheltered shallow water environments (e.g. estuarine tidal flats).
Suspension feeders are organisms that filter particles such as phytoplankton and detritus out of the water column. Because they rely on suspended particles, suspension feeders tend to be most abundant in environments with significant (tidal) currents and where the substrate is mainly sandy.[1]
Benthic infauna
Benthic infauna is the assemblage of organisms that live within or partly within the sediments of the seafloor. These animals range from gastropods (slugs, snails), amphipods (shrimp-like crustacea), polychaetas, and other invertebrates. Infaunal species often dominate communities of a soft substrate and are most abundant in subtidal regions. Although uncommon and few, infaunal species are also found to inhabit hard substrate communities, rock-boring clams being an example. Benthic communities, particularly the infaunal community, play an essential role in organic matter remineralization. In addition, infaunal species stimulate nitrogen cycling by extending the oxic-anoxic interfaces with more nutrients through burrow ventilation, particle reworking, irrigation, and excretion (see Nutrient cycling). Some meiofauna live in the oxygenated halo around burrows of large macrofauna. Some are long enough to extend into the oxic zone, and some tolerate anaerobic conditions but migrate to the oxygenated surface for gas exchanges.[8]
Benthic epifauna
The benthic epifauna are animals living on or attached to the seafloor. Corals, mussels, barnacles, echinoderms (starfish), and sponges are examples of these organisms. They occur in almost all substrates, but are most abundant and diverse in rocky intertidal areas and coral reefs. Epifaunal species play a vital role in the detritus-based food web as a link between primary producers and higher trophic-level predators.[3][8]
Phytobenthos

Microphytobenthos (microalgae, cyanobacteria) stabilize the seabed in shallow sheltered coastal environments by producing algal mats.[9] Algal mats can also be produced by macroalgae (seaweed Ulva spp.) in eutrophic coastal waters, often causing important nuisance.[10] Macrophytobenthos (seagrass meadows, mangroves, salt marshes) attenuate waves and currents and trap fine sediments, which are then held by root structures. Phytobenthos is a powerful natural ally in protecting the coast against erosion and enabling shore accretion and extension, see Nature-based shore protection.
Related articles
- Biogeomorphology of coastal systems
- Rocky shore habitat
- Coral reefs
- Seagrass meadows
- Mangroves
- Salt marshes
- Meiofauna of Sandy Beaches, soft sediments, and
- Deep sea habitat
- Estuarine ecosystems
References
- ↑ 1.0 1.1 1.2 Snelgrove, P.V.R. 2001. Marine sediments. Encyclopedia of Biodiversity, Volume 4, Academic Press, pp. 71-84
- ↑ 2.0 2.1 Satheesh, S. and El-Sherbiny, M.M. 2022. Ecology, distribution, and biogeography of benthos. Ecology and Biodiversity of Benthos, Elsevier, pp. 251-285
- ↑ 3.0 3.1 Lalli, C.M. and Parsons, T.R. 1997. Benthos. Biological Oceanography: An Introduction. Elsevier. pp. 177–195
- ↑ Kristensen, E. 1983. Ventilation and oxygen uptake by three species of Nereis (Annelida: Polychaeta). I. Effects of hypoxia. Marine Ecology Progress Series 12: 289-297
- ↑ 5.0 5.1 Sivapriya, V., Radhakrishnan, K. and Hussain, S.M. 2022. Benthos and its interaction with marine and estuarine ecosystem. In: Ecology and Biodiversity of Benthos, Elsevier, pp. 315-335
- ↑ Schokraie, E., Warnken, U., Hotz-Wagenblatt, A., Grohme, M.A., Hengherr, S., et al. 2012. Comparative proteome analysis of Milnesium tardigradum in early embryonic state versus adults in active and anhydrobiotic state. PLoS ONE 7(9), e45682
- ↑ Hayward, B.W., Grenfell, H.R., Reid, C.M. and Hayward, K.A. 1999. Recent New Zealand shallow-water benthic Foraminifera: Taxonomy, ecologic distribution, biogeography, and use in paleoenvironmental assessment. Institute of Geological and Nuclear Sciences Monograph 21, 258 p
- ↑ 8.0 8.1 Walag, A.M.P. 2022. Understanding the World of benthos: an introduction to benthology. Ecology and Biodiversity of Benthos, Elsevier, pp. 1-19
- ↑ 9.0 9.1 Cuadrado, D.G., Perillo, G.M.E. and Vitale, A.J. 2014. Modern microbial mats in siliciclastic tidal flats: Evolution, structure and the role of hydrodynamics. Marine Geology 352: 367-380
- ↑ Joniver, C.F.H., Photiades, A., Moore, P.J., Winters, A.L., Woolmer, A. and Adams, J.M.M. 2021. The global problem of nuisance macroalgal blooms and pathways to its use in the circular economy. Algal Res 58, 102407
Please note that others may also have edited the contents of this article.
|