Coastal and marine sediments
Definition of Sediment:
Natural unconsolidated granular material with density greater than water.
This is the common definition for Sediment, other definitions can be discussed in the article
|
Contents
Origin of coastal and marine sediments
Continental shelves are formed of sediment deposits that may reach thicknesses in excess of 1 km. These sediments have several origins:
Clastic sediments [1]
Clastic sediments (clast = fragment) are terrigenous materials eroded from land. They are ultimately weathering products derived from rock. About 85% of all coastal marine sediments are clastic sediments.
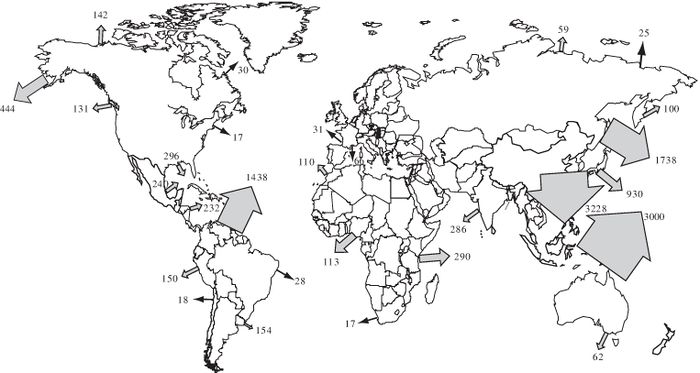
The products of rock weathering are transported mainly by rivers (for the present time indicated in Fig. 1), but also by wind and ice. The transported particles and fragments abrade each other and erode the surfaces over which they pass, thus contributing further to the breakdown of continental rocks. Time and distance are important factors: the longer the journey to the sea, the more chance there is for mineral grains to be rounded and reduced in size by abrasion, and sorted according to density and size and shape, and for chemically less stable minerals to break down. The most common solid products of weathering are rock fragments, quartz and clay minerals. Quartz is the only common mineral of igneous and metamorphic rocks that is both hard (resistant to abrasion) and chemically stable at the Earth's surface. This is the reason why quartz is the predominant mineral in present day beach and river sands and is also common in most ancient sandstones. The rate at which weathering occurs depends on climate, with rapid breakdown favoured by the high rainfall and temperatures of tropical areas and slow weathering occurring where water is absent or solid, i.e., in deserts and arctic zones.
A minor fraction of terrigenous material is deposited from the atmosphere. This wind-blown dust contains mainly very fine sand (quartz), but also silt and clay particles with appreciable proportions of aluminium (Al), iron (Fe), magnesium (Mg), and calcium (Ca).The flux of sediment in wind may be a minor factor in most areas today, except from our major deserts, e.g., the Sahara, but in the past it has been significant and has led to the accumulation of extensive loess deposits, e.g., in China. The exceptionally high sediment load of the Yellow river is due to erosion of these loess deposits, which are finally discharged into the Bohai Sea.
In arctic and subarctic areas and where mountains are sufficiently high to generate significant quantities of snowfall, glaciers are a major agent for sediment transport.
Clastic sediments can be classified according to grainsize, see Table 1. Grainsizes are indicative of the advancement of the weathering process; small grains derive from weathering of larger grains. Sediment samples generally contain grains of different sizes. Sedimentary deposits are named after the largest present grains (clasts). Common indurated sedimentary deposits are [3]:
- Breccia, Gritstone, Conglomerate: sedimentary rocks consisting of large clasts (boulders, cobbles, pebbles, gravel), cemented together by finer sediments. Breccia is composed of large angular elements and Gritstone of small angular elements; Conglomerate is composed of rounded elements.
- Till: indurated unsorted glacial sediment.
- Sandstone: sedimentary rock composed of sand-sized particles (mainly quartz) with finer grained material (feldspar, micas, lime, clay) that infills space in between, and cemented after deposition by minerals precipitated in the pores.
- Arkose: sandstone with a high content of feldspar.
- Greywacke: sandstone composed of poorly sorted angular grains (quartz, feldspar, small rock fragments).
- Flagstone: sandstone mainly composed of quartz and feldspar that is easily split in layers.
- Siltstone: sedimentary rock composed mainly of quartz, shell fragments and assorted heavy minerals.
- Claystone: sedimentary rock composed mainly of clay minerals and sometimes lime and organic matter.
- Shale: indurated form of siltstone or claystone; the fissility is due to the mica constituent.
- Marl: lime-rich mudstone.
| particle type | particle name | grainsize [mm] | indurated deposit |
|---|---|---|---|
| Gravel | Boulders | > 256 | Conglomerate, breccia,
gritstone, till |
| Cobbles | 64-256 | ||
| Pebbles | 4-64 | ||
| Granules | 2-4 | ||
| Sand | very coarse | 1-2 | Sandstone,arkose,
greywacke, flags |
| coarse | 0.5-1 | ||
| medium | 0.25-0.5 | ||
| fine | 0.125-0.25 | ||
| very fine | 0.0625-0.125 | ||
| Silt | 0.002-0.0625 | Siltstone, claystone,
mudstone, shale, marl | |
| Clay | < 0.002 |
Biogenic sediments [4]
Most biogenic sediments are erosion products of unconsolidated or consolidated marine detrital deposits or erosion products of framework-building organisms (e.g. coral reefs). These sediments, called "bioclastic sediments", are composed of fragments of organic skeletal materials . They mainly consist of calcium carbonate (CaCO3) in the form of calcite crystals or aragonite crystals. Other biogenic sediments, such as dead plant remains (peat), have a high organic component and are termed organic-rich biological sediments. Biogenic sediments may constitute a substantial fraction of the bed material in coastal environments where the supply of terrigenous material is small (no nearby river deltas). Many beaches in the tropics and subtropics consist almost entirely of carbonate sands which are derived from adjacent reefs composed of corals, skeletal material, shell fragments or precipitated calcium carbonate. Fine bioclastic sediments may also accumulate in sheltered coastal environments in temperate climate zones. An example is the inner part of the mega-tidal Baie du Mont Saint Michel (almost no fluvial sediment input), where tidal flats are composed of so-called tangue – low-cohesive fine sediment with a high biogenic content [5].
Chemical sediments
Chemical sediments form by precipitation of minerals out of solution as the water becomes saturated, which is often due to evaporation. The most common chemical sediments formed in this way are calcite (CaCO3), gypsum (CaSO4) and halite (NaCl). They are a common type of sediment of sabkhas, back-barrier coastal plains which are ubiquitous in arid climates.
Mineral composition of clastic sediments
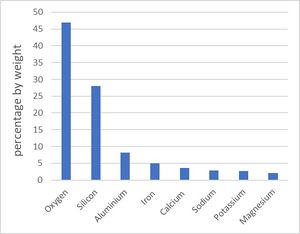
Figure 2 shows the average composition by weight of chemical elements in the lithosphere. These elements combine to form minerals. The chief minerals in the lithosphere (earth crust and uppermost mantle) are [6]:
| mineral | specific density |
|---|---|
| Quartz | 2.65 |
| Aragonite | 2.95 |
| Calcite | 2.71 |
| Orthoclase | 2.55-2.63 |
| Albite | 2.6-2.65 |
| Anorthite | 2.72-2.75 |
| Mica | 2.8-3.1 |
| Illite | 2.6-2.9 |
| Montmorillonite | 1.7-2 |
| Vermiculite | 2.4-2.7 |
- Feldspars: Aluminium silicates. The most common forms are: orthoclase KAlSi3O8, albite NaAlSi3O8 and anorthite CaAl2Si2O8. These minerals are fairly hard, often with pink, white, or grey in color. About 40% of the lithosphere consists of feldspars.
- Quartz: silicon dioxide crystal (SiO2);
- Mica: complex silicate minerals comprising elements K or Na and metal cations such as Al, Mg and Fe, that form crystals with a sheet-like arrangement of atoms;
- Clay: hydrous silicates that contain metal cations, generally aluminium. Most common clay minerals are kaolinite (Al2Si2O5(OH)4), illite, montmorillonite, vermiculite and bentonite. Their basic building blocks are sheets of silica (Si) tetrahedra and oxygen (O) and hydroxyl (OH) octahedra. Micas and clay minerals result from chemical weathering of igneous rocks and feldspars.
The specific densities (density relative to water) of these minerals is given in Table 2.
Physical and chemical properties of sediment
In this section several physical and chemical properties of coastal and marine sediments are shortly introduced. More detailed treatments can be found in other Coastal Wiki articles indicated in the text.
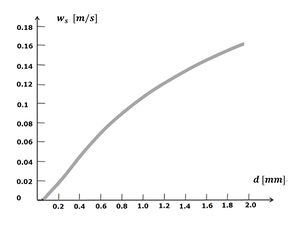
Fall velocity
The seabed of oceans and coastal waters are mainly formed by settling of sediment particles out of suspension. Which particles settle where is mainly determined by the particle fall velocity. The fall velocity of sediment particles depends on size and density. Because the density of different types of sediments is similar (Table 2), the grain size is determinant. The larger the grain size, the higher the fall velocity. The fall velocity as a function of the grainsize is displayed in Fig. 3. Indicated is the fall velocity of round quartz grains in still water at low concentration. In reality, sediment particles do not have a round shape, which slightly affects the fall velocity. The actual fall velocity in flowing water is very different, due to up and down motions caused by turbulent eddies. The concentration of sediment particles also plays a role. At very high concentration, the fall velocity decreases, because particles increasingly interfere with each other, a phenomenon that is called hindered settling.
Flocculation

If the fall velocity for very small particles, such as clay minerals, is derived from settling quartz spheres (Fig. 3), it seems so low that they almost never reach the bottom from suspension. The ubiquitous mud beds in coastal waters point to a different settling mechanism. This mechanism consists of flocculation. Clay minerals have a large surface area relative to their weight, and electrical surface charges. This ensures that they easily bind to one another and to other substances in the water. Clays are therefore classified as cohesive sediments. A substance that serves as a powerful binder is so-called EPS, extracellular polymeric substances [8]. These large organic molecules (polysaccharides, proteins, nucleic acids and lipids) are exuded by living organisms and therefore omnipresent in coastal waters. In addition to EPS, bacterial colonization can also play a role in flocculation [9]. Flocculation is further influenced by factors such as salinity and pH of the water. When flocs grow, they not only capture clay particles, but also other suspended sediments, such as silt and fine sand. Frequent encounters between sediment particles are important for floc growth. Flocculation is thus enhanced with a high concentration of suspended material and with a certain degree of turbulence. However, when turbulence is too strong, flocs break off again. Flocs settle much faster than the individual constituent particles - a factor of a thousand or more, see Fig. 4. The largest flocs are agglomerates of microfloc. These so-called macroflocs have the highest fall velocity, but they are less stable than the smaller microflocs. The various factors that play a role are captured in the following empirical formulas, for macroflocs and microflocs respectively [10]:
[math] w_{M}=\Large\frac{g B_M}{G}\normalsize \left(\Large \frac{c}{ \rho}\normalsize \right)^k \left(\Large \frac{G d_\mu^2}{\nu}\normalsize \right)^{0.33} \exp \left[-\left( \Large \frac{u_{*M}}{\sqrt{\tau / \rho}}\normalsize \right)^{0.463} \right] [/math]
[math] w_{ \mu}= \Large \frac{g B_\mu}{G}\normalsize \left(\Large \frac{Gd^2}{\nu}\normalsize \right)^{0.78} \exp \left[-\left(\Large \frac{u_{\mu}}{\sqrt{\tau / \rho}}\normalsize \right)^{0.66} \right] , [/math]
where the index [math]M[/math] designates the macroflocs and the index [math]\mu[/math] the microflocs. Other symbols stand for: [math]d[/math] the grainsize of the constituent particles, [math]d_{\mu}[/math] the grainsize of the constituent microflocs, [math]\tau=\rho u_*^2[/math] the bed shear stress and [math]c [/math] the suspension concentration in [math] kg/l [/math]. The velocity shear rate [math]G[/math] is given by
[math] G = \sqrt {\epsilon / \nu } , [/math]
where [math]\epsilon[/math] is the energy dissipation rate per unit mass and [math]\nu [/math] the kinematic viscosity. For the Tamar and Gironde estuaries the following parameter values were established: [math] B_M = 0.13, \; B_{\mu} = 0.6, \; k = 0.22, \; u_{*M} = 0.067 m/s, \; u_{*\mu} = 0.025 m/s, \; d_\mu = 10^{-4} m, \; d = 10^{-5} m [/math]. The settling velocities observed in the Tamar and Gironde are 0.5-1 mm/s for microflocs and about 5 times larger for macroflocs.
See also: Mud, Sediment deposition and erosion processes, Dynamics of mud transport.
Sediment deposits in coastal waters
A more detailed introduction to sediment deposition and erosion is given in the article Sediment deposition and erosion processes.
Deposition of sediment on the seabed takes place when conditions are suitable. These conditions are different for each type of sediment.
When a sediment-laden water mass reaches an area of lower flow strength and wave activity, part of the carried material is deposited. Sediment particles with the greatest fall velocity settle first and particles entrained as bedload (rolling and jumping along the bottom) come to rest. When the current strength and wave activity further decrease, the fine suspended material also settles. Most sedimentation takes place in the period around slack tide (flow reversal). Part of the deposited material will afterwards be resuspended by the recovering tidal flow, but another part will remain. This can lead to temporary or permanent deposition. Deposits are temporary if they are insufficiently consolidated and re-eroded during conditions of strong currents (e.g., spring tide) or strong wave action (storm). Permanent deposits have a layered character; each layer represents a deposition period. Successive layers may contain different types of sediment if they have been deposited under different conditions.
Bedforms
Sediment layers are generally horizontal, but can also have a wavy character. These undulations are caused by the fact that the interaction between flow and sediment bed does not behave linearly, meaning that small disturbances of the flat sediment bed can grow exponentially. This leads to the emergence of a large range of bedforms, as explained in the article Stability models. The smallest bedforms, ripples, arise in places where bed sediments are sandy and where currents and wave activity are not very strong but sufficient to set sediment in motion. This is explained in the articles Wave ripples and Wave ripple formation, for situations where sediment movement is mainly determined by waves. Bed ripples exert friction on tidal currents and have a great influence on the flow velocity. More details can be found in the articles Bedforms and roughness and Bed roughness and friction factors in estuaries. In addition to ripples, much larger bedforms can also arise: megaripples and dunes (also called sandwaves) with wavelengths ranging from several meters to more than hundred meters. In underlying sediment layers, bed ripples are usually not preserved, but megaripples and dunes can generally be observed quite well.
Bedforms do not occur with deposits of fine cohesive material (mud). Dewatering of freshly deposited mud is a slow process and mud layers therefore remain fluid for a long time as so-called fluid mud, see for example the article Dynamics of mud transport. Once consolidated, the erosion resistance is high, so that no bed ripples can form. Mud layers therefore have a smooth surface and exert little friction on the flow.
Graded sediment
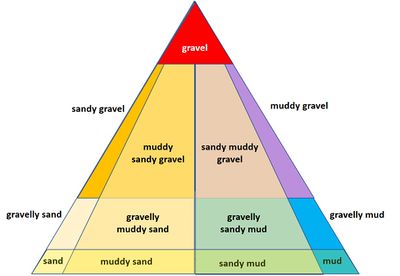
Sediment deposits in coastal areas are not always layered; mixed deposits occur frequently. These deposits are referred to as graded sediment. Layered sediment deposits can be mixed by wave action, although this reduces the content of fine soil material. Mixing also takes place through bioturbation: soil animals (mainly worms and molluscs) bring material from deeper layers to the surface through ingestion and excretion [12][13]. Deposition of mixed sediment also takes place by excretion of filter feeders in the form of fecal pellets. Terminology for mixed deposits is indicated in Fig. 5 for different mixtures of gravel, sand and mud. Mud is itself a mixture of particles with a grain size of less than 0.063 mm, consisting of very fine sand, silt, clay and organic matter.
See also: Interpolation of measured grain-size fractions, Biogeomorphology of aquatic systems, Sandy shore habitat, Meiofauna of Sandy Beaches.
Spontaneous segregation of graded sediment can occur under certain conditions. This segregation generates contiguous patches of different types of sediment. The underlying feedback mechanism links sedimentation of fine material on a smooth muddy seabed to a locally reduced degree of turbulence [14]. As a result, fine material will mainly deposit in places where fine material already dominates on the seabed, so that these muddy patches increase in size and in mud content. This continues until no fine material is available any more from neighboring patches of coarser deposits from which the fines have been winnowed.
Sediment transport
The processes that underlie sediment transport are strongly related to turbulent flow structures in a thin boundary layer near the bed and above. Because these processes are very complicated and not even fully understood, empirical formulas are used in practice for the description of sediment transport. These formulas are described in the articles Sand transport and Sediment transport formulas for the coastal environment. These articles deal mainly with non-cohesive sediments. Transport of cohesive sediments is dealt with in the articles Dynamics of mud transport and Sediment deposition and erosion processes. For measurement of sediment transport see: Measuring instruments for sediment transport, Laboratory and in situ analysis of samples.
Sediment contamination
As indicated earlier, fine sediments - clay particles in particular - can easily bind with other substances in the water. This binding is called sorption: adsorption (=surface binding) or absorption (=uptake). This property allows fine sediments to filter out dissolved contaminants from the water. The water is less polluted, but the contamination of bed sediments, on the other hand, increases. The partition of pollutants between the dissolved phase and the sediment-bound phase is represented by the parameter [math] K_d [/math]. This partition, i.e. the value of [math] K_d[/math], depends on the type of pollutant and the type of sediment. Laboratory tests show that for inorganic contaminants, for example heavy metals such as lead (cation Pb2 +), cadmium (Cd2 +) and copper (Cu2 +), the partition depends on the sediment grainsize [15]. The smaller the grainsize, the stronger is the sorption (large [math] K_d [/math]), independently of the concentration of dissolved heavy metals. This does not apply to organic contaminants such as PCBs (polychlorinated biphenyls) and PAHs (polyciclic aromatic hydrocarbons), the sorption of which is determined by the organic carbon content in the sediment [16]. This is a well-known feature in water sanitation technology, where active carbon is used to filter contaminants from the water.
Contaminants are much less toxic to marine organisms when they are bound to sediment than when they are dissolved in water [17]. The bioavailability even decreases as the sediment bed gets older. Perturbation of the sediment bed by dredging or by bioturbation, however, plays a role. When sediment is worked up from a deeper anoxic layer to a higher oxic layer, attached metals are released by desorption. Burial from sediments to deeper soil layers, on the other hand, reduces bioavailability. The bioavailability of contaminants in soils can be reduced by adding active carbon.
Spatial distribution of sediments
The type of sediment occurring on the seafloor varies greatly from place to place. The presence of nearby sediment sources plays an important role, but hydrodynamic conditions strongly influence which sediments are deposited where. Because certain types of sediment are only deposited under specific conditions, sorting occurs of the different types of sediment that are supplied. In the following, a short description is given of resulting sediment deposits in different marine environments.
Oceans
The deep oceans have four main sediment sources [4]:
- Clastic fluvial sediments. Large quantities of fine sediments (silt, clay and fine sand) are deposited by the world's major rivers at the edges of the continental shelfs (see Fig. 1). These deposits destabilize the continental shelf slope, causing the sediment to slide down to the ocean floor by slumping or through gravity currents. These deposits are confined along the shelf break and are not further spread over the ocean floor.
- Atmospheric dust. A large part of the fine sediments entrained by wind from arid land areas is deposited in the oceans (e.g., red clay deposits, manganese nodules). This contribution to ocean sedimentation is relatively small nowadays.
- Carbonaceous ooze. The remains of calcareous algae, mainly coccoliths and foraminifera, are a major component of the seafloor sediment in large parts of the ocean. Below depths of 3 to 4 km, however, they do not occur, because the scales dissolve here as a result of too high a pressure and too high a concentration of carbon dioxide.
- Siliceous ooze. The remains of silicate plankton, mainly diatoms and radiolaria, dominate in other parts of the ocean - especially in the central part of the Pacific and in the Antarctic Ocean.
Beach and foreshore
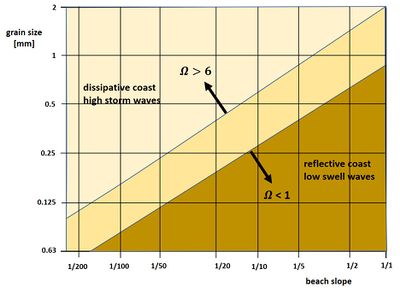
Sediments on the foreshore and subaerial beach are derived from nearby rivers or from ancient offshore river deposits. The foreshore is a highly energetic environment due to the activity of waves, which prevent settling of fine sediments. Sand and gravel are the dominant seabed sediments, depending on the supply of these sediments. The coarsest sediments are found in the surf zone, which can extend during storm conditions over a large part of the otherwise dry beach. The finer fractions are found in the offshore zone and also on the backshore and the dunes, which are fed from the dry beach by aeolian transport. The type of sediment on the shoreface largely determines the equilibrium shoreface slope for a given wave climate. The relationship between sediment grain size, wave climate and shoreface slope is characterized by the Dean parameter
[math]\Omega = H / (w T) , [/math]
where [math]H[/math] is the significant offshore wave height (before breaking), [math]w[/math] the sediment fall velocity and [math]T[/math] the peak wave period. This relationship is schematically shown in Fig. 6. For a more detailed discussion of the shoreface equilibrium profile the reader is referred to the article Shoreface profile.
Estuaries
The sediment distribution in estuaries is particularly complex. Sediment deposits depend on the supply of river sediment, the supply of sediment from the sea and on local flow and wave conditions, in relation with a generally intricate topography. In addition, deposits are influenced by strong fluctuations in river discharge, tides and wave conditions at various timescales. Nevertheless, the sediment distribution in estuaries generally displays a characteristic pattern that is shown in Fig. 7 and discussed below.
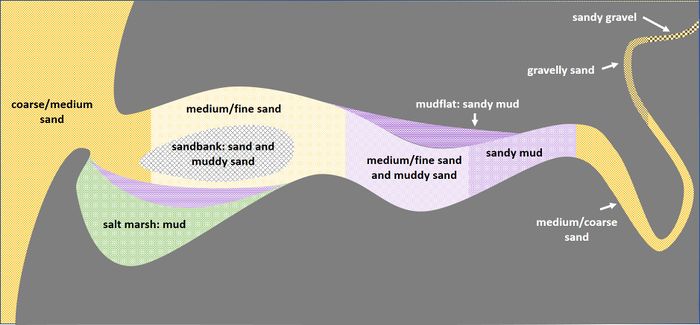
Gravel transported by rivers is mostly deposited upstream and does not reach coastal plain estuaries; rivers discharge mainly sand and mud into these estuaries. Sand and mud are also imported from the sea by tidal flood currents. The estuarine morphology is in morphodynamic equilibrium if, averaged over a long period, the inflow of sand and mud is balanced by export to the sea by outflowing ebb currents, see Morphology of estuaries. This is not a static equilibrium, because sand and mud are moving around the estuary all the time, including temporal deposition and erosion.
Morphodynamic equilibrium does not imply that the concentration of suspended sediment in an estuary is uniform. Concentration maxima occur in convergence zones where temporal settling of sediments takes place. These convergence zones depend not only on flow strength and flow pattern, but also on transport, settling and erosion characteristics of sediment particles. The areas of highest suspended concentration and deposition thus differ for sand and mud [20].
Convergence of sand transport takes place on the outer delta, where the ebb flow expands and decelerates. Within the estuary, convergence zones of sand transport are marked by sand banks and sand bars which have grown at the junctions of ebb and flood flow channels and at the inner bends of flow meanders.
Convergence of mud transport (silt and clay) occurs in the transition zone between flood dominant near-bed flow (due to tidal asymmetry and estuarine density-driven circulation) and ebb dominant flow (in combination with river flow). This zone has the highest mud concentrations, both in the water column (turbidity maximum) and in the channel bed. Besides, mud concentrations are high in sheltered areas where fine sediments can settle without immediately being resuspended, such as mudflats and salt marshes.
See also: Estuarine ecosystems.
References
- ↑ 1.0 1.1 1.2 Huggett, R.J. 2007. Fundamentals of geomorphology. Routledge, Taylor & Francis
- ↑ Milliman, J. D. and Meade, R. H. 1983. World-wide delivery of sediment to the oceans. Journal of Geology 91, 1–21
- ↑ Selley, R.C. 2005. Sedimentary rocks: Mineralogy and Classification. In: Encyclopedia of Geology (Editors: Richard C. Selley, L. Robin M. Cocks and Ian R. Plimer). Elsevier.
- ↑ 4.0 4.1 Taylor, K.G. 2008. Sediments and sedimentation. In: An Introduction to Physical Geography and the Environment (J. Holden, editor), Pearson Education Limited
- ↑ Desguée, R., Robin, N., Gluard, L., Monfort, O., Anthony, E.J. and Levoy, F., 2011. Contribution of hydrodynamic conditions during shallow water stages to the sediment balance on a tidal flat: Mont-Saint-Michel bay, Normandy, France. Estuarine, Coastal and Shelf Science, 94, 343-354
- ↑ Anderson, R.S. and Anderson, S.P. 2010. Geomorphology: The Mechanics and Chemistry of Landscapes. Cambridge University Press. p. 187.
- ↑ Migniot, C. 1968. A study of the physical properties of various forms of very fine sediments and their behaviour under hydrodynamic action. La Houille Blanche 7, 591–620
- ↑ Grabowski, R.C., Droppo, I.G. and Wharton, G. 2011. Erodibility of cohesive sediment: the importance of sediment properties. Earth Science Reviews 105 (3-4): 101-12
- ↑ Linley, E.A.S. and Field, J.G. 1982. The nature and significance of bacterial aggregation in a nearshore upwelling ecosystem. Est.Coast.Shelf Sci. 14: 1-11
- ↑ Dyer, K.R. and Manning, A. J. 1999. Observation of the size, settling velocity and effective density of flocs and their fractal dimension. J.Sea Res. 41: 87-95
- ↑ Blair, T.C. and McPherson, J.G. 1999. Grain-size and textural classification of coarse sedimentary particles. Journal of Sedimentary Research 69: 6–19
- ↑ Baumfalk, Y.A. 1979. Heterogeneous grain size distribution in tidal flat sediment caused by bioturbation activity of Arenicola marina (polychaeta). Netherlands Journal of Sea Research 13: 428-440
- ↑ Gallagher, E.D. 2008. Bioturbation. Biol. Ocean. Processes, EEOS 630
- ↑ Murray, A.B. and Thieler, E.R. 2004. A new hypothesis and exploratory model for the formation of large-scale inner-shelf sediment sorting and ‘rippled scour depressions’. Continental Shelf Res. 24: 295-315
- ↑ Schorer, M. 1997. Pollutant and organic matter content in particle size fractions. Freshwater contamination, IAHS publ. 243.
- ↑ Karickhoff, S.W., Brown, D.S. and Scott, T.A. 1979. Sorption of hydrophobic pollutants on natural sediments. Water Research 13: 241-248
- ↑ National Research Council. 2003. Bioavailability of Contaminants in Soils and Sediments: Processes, Tools, and Applications. Washington, DC: The National Academies Press. doi: 10.17226/10523
- ↑ Flemming, B.W. and Fricke, A.H. 1983. Beach and nearshore habitats as a function of internal geometry, primary sedimentary structures and grain size. In: McLachlan, A., Ersamus, T. (Eds.), Sandy Beaches as Ecosystems. Dr. W. Junk Publishers, The Hague, pp. 115–132
- ↑ Dalrymple, R.W., Zaitlin, B.A., Boyd, R., 1992. Estuarine facies models: conceptual basis and stratigraphic implications. Journal of Sedimentary Petrology 62, 1130–1146
- ↑ Dronkers, J. 2017. Dynamics of coastal systems. World Scientific Publ. Co., 753 pp.
Please note that others may also have edited the contents of this article.
|