Seaweed (macro-algae) ecosystem services
Although seaweeds may look like plants at first glance, they are not. Seaweeds are algae; they lack the various structures characteristic of land plants, such as roots, leaves and other organs found in vascular plants. Their body is called 'thallus' and the leaf-like structures 'lamina'. They often have a stipe (a stem-like structure) and a holdfast that allows them to attach to a substrate. Seaweeds are phototrophs, deriving their energy from photosynthesis and live in shallow seawater where light can penetrate. There are many different types of seaweed. They have been cultivated by humans for a long time because of the many ecosystem services they deliver. Kelps, large brown algae of the order Laminariales, are a major group of seaweed, discussed in the article Kelp forests.
Contents
Seaweed species
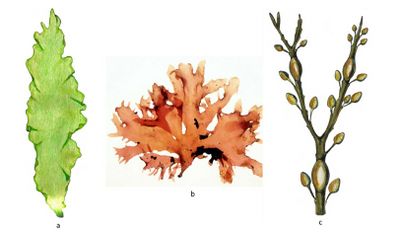
Seaweeds are broadly classified into three taxonomic groups[1][2]:
- Brown seaweeds belong to the phylum Ochrophyta, and they are all in the class Phaeophyceae (around 2 000 species); their pigments are chlorophylls a and c and carotenoids (where fucoxanthin predominates, responsible for their brownish color). Brown seaweed cultivation has concentrated on two cold-water genera: mainly Laminaria/Saccharina (also known as kelp) and Undaria (also known as wakame). Among North Atlantic kelp species Saccharina latissima is the fastest-growing macroalgal species[3]. In 2019, brown seaweeds accounted for 47.3 percent of world seaweed cultivation in terms of tonnage and 52 percent in terms of value. Most species within the class Phaeophyceae are predominantly cold-water organisms that benefit from nutrients upwelling, but the genus Sargassum appears to be an exception. Numerous Sargassum species are distributed throughout the temperate and tropical oceans of the world, where they generally inhabit shallow water and coral reefs, and the genus is widely known for its planktonic (free-floating) species.
- Red seaweeds belong to the phylum Rhodophyta (over 7 200 species); they have chlorophyll a, phycobilins, and some carotenoids as photosynthetic pigments. Red seaweed cultivation is concentrated on two warm-water genera, Kappaphycus/Eucheuma (mainly in Indonesia) and Gracilaria (mainly in China) and one cold-water genus (Porphyra, also known as nori). In 2019, red seaweeds accounted for 52.6 percent of world seaweed cultivation in terms of tonnage and 47.6 percent in terms of value.
- Green seaweeds are included in the phylum Chlorophyta (more than 1 800 macroalgae species), and their pigmentation is identical to that of vascular plants (chlorophylls a and b and carotenoids). Cultivation of green seaweeds has been small and on a downward trend since the early 1990s.
Typical examples of the three taxonomic groups are shown in Fig. 1.
A more detailed description of macroalgae species is given in the article Diversity and classification of marine benthic algae.
Growth conditions
Environmental conditions crucial for seaweed ecology are:
- Seawater (or at least brackish water).
- Sufficient light to support photosynthesis.
- Temperature. Most seaweed species in the warm-temperate Atlantic-Mediterranean region thrive at temperatures between 10 and 25 oC, with optimum growth and reproduction between 15-20 oC. [4]
- An attachment point. Most seaweed species inhabit the littoral zone (nearshore waters) and within that zone, on rocky substrate (bedrock, boulders, cobbles or biogenic structures), while some others are able to attach to soft sediments (e.g. Halimeda, Caulerpa). There are few genera (e.g., Sargassum and Gracilaria) which do not live attached to the sea floor, but float freely.
Reproduction
Seaweed species have various reproduction strategies[1]. Some seaweed species can reproduce asexually through one of the following processes: (1) by thalli fragmentation, in which each piece originates a new thallus and reconstitutes a new individual; (2) by means of propagules, that is, through a small cluster of cells, with the ability to attach to a substrate and functionally originate a new thallus; (3) by spores released to the outside of the sporocysts (specialized structures, originating from modified mother cells) through an opening in their wall. Other seaweed species reproduce sexually, which involves the production of specialized cells through meiosis, the gametes, formed inside a mother cell or gametocyst. Most cultivated seaweeds reproduce sexually (e.g. kelps and the red algae Porphyra spp.). Clonal propagation (e.g. in the red alga Kappaphycus) and recourse to a limited number of parent genotypes (e.g. in kelp) account for the production of commonly cultivated seaweeds. The resulting impoverishment of genetic diversity increases seaweed susceptibility to diseases and decreases their fitness within their cultivation environment[5].
Occurrence
Wild seaweed populations occur globally in all coastal areas and occupy about 7 million km2 [6]. About 1.1 million tonnes is harvested; harvests from wild populations are affected by overexploitation and climatic change. World seaweed production is primarily supported by aquaculture with a yield of about 35 million tonnes in 2019, which accounted for 97 percent of world seaweed production[2]. There is a strong regional imbalance in seaweed production. In 2019, seaweed production in Asia (99.1 percent from cultivation) contributed 97.4 percent of world production, and seven of the top ten seaweed producing countries were from Eastern or South-eastern Asia (China, Indonesia, Philippines, Koreas, Japan, Malaysia). Current seaweed aquaculture (2019) occupies only about two thousand km2, while ca. 48 million km2 of the oceans are suitable for seaweed aquaculture[7].
Sea lettuce, stranding by hundreds of thousands of tons on beaches in Europe, USA and China, are a major nuisance since the 1990s. These so-called 'green tides', consisting of blooms of the green macroalgae genus Ulva, are a consequence of coastal eutrophication, especially from agricultural and aquacultural effluents. If not removed in time, the algae can turn into a stinking morass, which can produce toxic hydrogen sulphide from its anoxic interior and have major detrimental effects on the affected coastal ecosystems[8]. 'Golden tides', made up of blooms of the genus Sargassum found in the Gulf of Mexico, are a major tourist nuisance on beaches in the Caribbean. Parts of the West African coastline are also affected. Green and golden tides can be turned into profit by harvesting the macroalgae and converting them into useful compounds (e.g., bioactive compounds, food, biofuels)[9].
Macro-algae are sensitive to marine pollution, disease outbreaks, epiphytic infestation, algal parasites, periodic storms, and ocean warming. The responses of aquacultured seaweeds to ocean acidification vary by species. Several economically important seaweeds of the genera Gracilaria and Porphyra respond to ocean acidification with an increased growth rate, while several other species are capable to acclimatize to elevated CO2 levels[10].
Ecosystem services

Seaweeds are valuable due to their high content in compounds with different biological activities, including complex organic compounds and primary and secondary metabolites, such as phytopigments (xanthophylls and carotenoids), polyunsaturated fatty acids (PUFAs) comprising docosahexaenoic acid (DHA), phenolic compounds, tannins, peptides, lipids, enzymes, vitamins, carbohydrates, terpenoids, and others[12].
More than 80% of macroalgae production and harvest is currently destined for direct human consumption or as hydrocolloids (thickeners, gelling agents, etc.)[13]. Of the more than 15,000 species of seaweeds, 34 species are suitable for aquaculture, 145 species for human consumption and 101 for hydrocolloids extraction[14]. Being mostly low-value commodities, seaweed aquaculture accounted for just 5.4 percent of the $275 billion of global aquaculture sale value in 2019[2]. Yet seaweeds are an important provider of ecosystem services. The current use of seaweed represents only a small fraction of the ecosystem services that seaweed can potentially provide worldwide.
Numerous advantages makes seaweed a potential candidate for the production of biomaterials and biofuels, such as (1) the use of water as an electron donor to perform oxygenic photosynthesis, (2) very high per-acre biomass productivity compared to oily seed crops, (3) ability to resolve food vs fuels debates as they are a nonfood feedstock, (4) no requirement for arable and productive agricultural land for cultivation, (5) adaption to growth in brackish water, seawater, and wastewater, and (6) production of a diverse range of products[15]
For a cost-efficient production of biomaterials and biofuels, the seaweed biorefinery concept using microbial conversion processes should be applied. Lack of lignin in seaweeds implies that the harsh pre-treatment applied for release of fermentable sugars from terrestrial lignocellulosic biomass is not required. The complex carbohydrates laminaran and mannitol (the reserve food of brown algae) can easily be extracted by hydrolysis[3].
Current and potential uses
The ecosystem services (potentially) provided by seaweeds are numerous. Sea also the article Diverse applications of macroalgae.
- Food and food additives. Seaweeds used as human foods tend to be more valuable than those employed for industrial applications. Macroalgae have a protein content that can range from 7 to 31% of dry weight and a lipid content ranging from 2 to 13% of dry weight. They also contain a considerable amount of carbohydrates (up to 32–60% of dry weight). Regarding the macroalgae content in micronutrients, they are a good source of vitamins, especially of the B-group representatives (i.e., B1, B12), as well as the lipophilic vitamins A and E[12]. Cultivated brown seaweeds are mostly used as human foods (e.g. kombu soup and wakame salads) as well as abalone feed. In 2019, the 11.7 million tonnes of world production of carrageenan-containing seaweeds (carrageenophytes) was almost entirely supplied by Kappaphycus/Eucheuma cultivation in tropical areas. Similarly, the 3.7 million tonnes of world production of agar-containing seaweeds (agarophytes) in 2019 was almost entirely supplied by Gracilaria, mostly provided by cultivation. Japanese kelp (Laminaria japonica) was one of the earliest raw materials for producing monosodium glutamate, which is widely used as a flavour enhancer for umami taste. Agar extracted from Gracilaria and other agarophytes, carrageenan extracted from Kappaphycus/Eucheuma and other carrageenophytes, and alginate extracted from Laminaria/Saccharina and other brown seaweeds are seaweed-based (hydro)colloids widely used as food additives to enhance the quality of a variety of foods and able to form gelatins at room temperature[2].
- Nutraceuticals. Seaweed extracts, such as iodine, fucoidan, fucoxanthin and phlorotannins, are used as food supplements for health benefits[2]. Although about 50% of the dry weight of macroalgae is carbohydrates, humans do not have the necessary enzymes to break these long molecules, so they are not absorbed by the digestive system, behaving like water soluble fibers. Macroalgae are extraordinary dietary supplements due to their high content of minerals, vitamins, and structural polysaccharides (fibers), which can facilitate intestinal transit and lower the cholesterol level in the blood. Multiple health benefits of seaweed consumption (e.g. improving gut health and reducing the risks of non-communicable diseases such as obesity and Type II diabetes) have been demonstrated by a large body of published research[1].
- Mitigation of atmospheric CO2. Seaweed communities of the North Atlantic coasts have an annual productivity of approximately 2 kg C /m2/yr, which is far higher than, for example, temperate tree plantations or grasslands with a productivity of generally less than 1 kg C /m2/yr [3]. There is mounting evidence for seaweeds’ role in sequestering carbon, particularly as it relates to farmed production (although there is disagreement whether wild macroalgae meet the criteria of the Blue Carbon framework, see Blue carbon sequestration). Seaweed sequestration companies could increase their scale and sell carbon offsets for emitters[7]. Blue carbon sequestration by sinking large amounts of farmed seaweed biomass into the deep ocean is discussed in the article Blue carbon sequestration.
- Carbohydrates: Sugars (glucose, rhamnose, xylose and glucuronic acid, etc.) in green seaweed biomass, algin, alginate, cellulose, mannitol, etc. in brown seaweed and cellulose, agar, carrageenan, starch, xylan, mannan, fructan, etc. in red algae[16]. The hydrolysates that comprise fermentable sugars can be further decomposed through the appropriate fermentation process by microorganisms to generate various bio-products: butanol, 1,2-propanediol, pyruvic acid and lactic acid from green algae; butanol, 2,3-butanediol, 2,3-butanediol and CH4 from brown algae; butyric acid from red algae. Because of the high water content of macroalgae, hydrothermal liquefaction is an advantageous way to extract biofuels[17]. Hydrothermal liquefaction (possibly using catalysts) decomposes macroalgal biomass into biofuels at temperatures between 250-400 °C and high pressure. Ethanol can be extracted from all algae for the production of biodiesel. Seaweed farming covering a marine area of about the size of the country of Luxembourg, would be able to provide about 6 million tonnes carbohydrates. With current 90% enzymatic conversion into ethanol this would yield close to 2 billion litres of bioethanol, which could cover about 50% of the EU’s ethanol demand[3].
Biosynthesis of value-added products (e.g. butanol) can be optimized through the application of genetic modification techniques, using the CRISPR system[16].
- Pharmaceuticals, cosmetics. Currently, use of macroalgae is oriented toward the production of phycocolloids (e.g. agar) which have a preponderant role in the pharmaceutical industry due to their stabilizing properties, as thickeners, in the extraction of compounds with antiviral, antibacterial, or anti-tumoral action. Carrageenans are used in medicine as they can inhibit the development of the herpes virus and infection by the human papilloma virus. They are further useful in the treatment and washing of hair due to their ability to bond with keratin. Alginic acid is a complex polysaccharide extracted from brown algae, used in the medical and cosmetic industries because of its stability in wide ranges of pH and salinity, thus allowing fast healing and neutralization of certain heavy metals or in cases of intoxication by ingestion. Green macroalgae have been used as anti-worms. Red macroalgae are used as anticoagulants, anti-worms, and in the treatment of gastritis and diarrhea, while brown macroalgae are commonly used in menstrual disorders, hypertension, skin diseases, syphilis, and gastric ulcers and have an anticoagulant effect[1]. The glycolipids contained in seaweed are essential components of the cellular membrane. Several studies conducted on different glycolipids from seaweed show their anti-inflammatory and antiproliferative effects[12]. Many other compounds sourced from marine algae, including phenolic compounds, flavonoids, terpenoids, caretenoids, alkaloids, phlorotannins, bromophenols, amino acids, peptides, proteins, polysaccharides, and fatty acids have anti-inflammatory and immunomodulatory effects[18]. Fucoidan extracted from brown seaweeds (generally kelp species) is used in a range of therapeutic health care preparations, being incorporated as high value ingredients in nutritional, medical device, skincare and dermatological products. Ulvan, extracted from Ulvales green algae, is a sulfated polysaccharide with many applications in agriculture and medicine, due to its immunomodulating, antiviral, antioxidant, antihyperlipidemic, and anticancer properties[19]. The development of algae-derived pharmaceuticals has to deal with the natural variability inherent in the production of bioactive compounds by marine algae. This may require strict control of cultivation conditions or the design of synthetic analogs with manageable properties[18].
- Eutrophication reduction. By extracting nutrients (nitrogen and phosphorus) from surrounding waters, the photosynthetic process of seaweeds can mitigate eutrophication[20].
- Phosphorus retrieval. Currently run-off from land washes nutrients into the rivers (causing algae blooms) and via coastal waters ultimately into the deep ocean. Phosphate rock is a non-renewable fertilizer ore that is in diminishing global supply and for which there is no substitute. Large-scale seaweed cultivation in inshore and nearshore seas could prevent the loss of phosphates from run-off as these macroalgae have a high uptake rate of these nutrients. [3].
- Water treatment. Removal of organic pollutants, heavy metals and pathogens due to the symbiotic system of seaweeds and bacteria.
- Mitigation of ocean hypoxia. There are an estimated 250 eutrophic sites around the world where offsetting seaweed could help raise pH and provide oxygen to the aquatic ecosystem, thereby locally reducing ocean acidification and deoxygenation. There are over 500 sites of hypoxic events that could also benefit from improved water quality (oxygen and reduced nutrient load)[7].
- Biochar, a substance with many industrial applications, is obtained by heating seaweed biomass in the absence of oxygen (pyrolysis). Biochar increases the fertility of acidic soils; it increases agricultural productivity, thus reducing the need for fertilizers.
- Biofertilizer/biostimulants. Macroalgae can be used as a natural fertilizer in agriculture, minimizing the need for chemical fertilizers and avoiding associated eutrophication. Seaweed-based biofertilizer or biostimulants improve soil conditions and potentially reduce agricultural pesticides. Some red algae of the family of Corallinaceae are used to correct the pH of acidic soils, while at the same time they increase the production of crops because they contribute certain elements such as magnesium, strontium, boron, and iron.
- Mitigation of plant stresses. Some seaweed extracts increase salt stress tolerance, drought tolerance and freeze tolerance and resistance to insects and pathogens. It increases soil fertility, mycorrhizal associations and root branching. This results in increased plant vigor and growth, delayed senescence, germination, flowering and higher yield[21].
- Integrated multitrophic aquaculture (IMTA). Finfish and crustacean production results in some of the largest CO2 emissions in the aquaculture sector. Growing seaweeds alongside aquatic animal production could become an ‘‘offset pre-requisite’’ for finfish or crustacean farms and permitting in some regions around the world[7].
- Biodegradable goods and packaging. Polyhydroxyalkanoates (PHAs) are a kind of environment-friendly polyesters existing in some specific bacteria, and can be used for synthesizing biodegradable plastics, making itself become a type of green biomaterial as a potential substitute for petroleum plastics.
- Provision of habitats for fish and other marine organisms. The nursery area provided by wild seaweed forests is as important as the similar function provided by seagrass in both temperate and tropical seascapes[22].
- Shore vegetation. Driftweed (seaweed washed ashore) can play a role in sand dune development by enabling pioneering salt tolerant plants to establish along the driftline, see Shore protection vegetation. It also provides food and shelter for invertebrates, which in turn act as a food source for other species. There is some evidence that seaweed also plays a role in shore protection by damping of wave energy[23][24].
- Mitigation of overfishing through providing alternative livelihoods to fishing communities.
- Provision of animal feeds.
- Employment. Seaweed cultivation is labour intensive; it employs many part-time or occasional workers. A large portion of the USD 14.7 billion of first-sale value of farmed seaweed are wages or incomes that support the livelihoods of numerous households in coastal communities. Activities further downstream (e.g. post-harvest handling, distribution, processing and marketing) tend to generate more income and employment[2].
Impediments

- High costs. Existing cultivation and harvesting technology is labour intensive and needs to be optimised to reduce costs and energy demand. A major bottle-neck in mass cultivation of kelp biomass in the sea is the production of juvenile sporophytes on ropes from zoospores via female and male gametophytes[3] (Fig. 3).
The high cost of synthesizing biodegradable plastics, especially the substrate, is one of the bottlenecks for large-scale industrialization.
- Low profits of seaweed farmers. Low profits are due to low and fluctuating market prices, high shipping costs, the dependence of farmers on processors for the procurement of their farming materials and their lack of farm-management skills. This is exacerbated by suboptimal practices (e.g. premature harvesting) owing to financial constraints or unstable market conditions, low quality due to inappropriate post-harvest handling and lack of value addition[2].
- Food safety issues related to pollution of nearshore waters. Seaweeds are efficient nutrient extractors and may accumulate compounds that pose harm to human health[25]. Toxicity existing in the raw materials limits the economic value.
- Integrated multitrophic aquaculture (IMTA). Finfish farmers tend to lack incentives to integrate seaweeds into their farming systems, as they are facing more complex farm management without benefiting from the positive impacts of seaweeds on water quality (e.g. increasing the number of fish allowed to be reared)[26].
- Climate change. Farming environments are deteriorated by rising seawater temperatures, increasing extreme weather conditions, more voracious grazing of predators and more frequent and severe disease outbreaks[2]
- Diseases. Seaweed farming and culture are seriously affected by diseases. Ice-ice disease has impacted the farming of the kappa-carrageenan-producing Kappaphycus alvarezii, commercially called 'cottonii'. Significant diseases affecting cultivated kelps (e.g., Saccharina japonica) include green-rot, white-rot, blister disease, which may be environmentally induced, and malformation disease of summer sporelings and swollen stipe or 'frond twist disease' which are caused by bacteria. Parasites such as Pythium, an oomycete fungus, causes red rot or red wasting disease in the red seaweed Pyropia commonly used in making sushi[27].
- Competition for space in nearshore waters.
Environmental issues
Improperly managed seaweed cultivation can negatively affect the environment or ecosystem, especially under poor siting and management practices, through [2]:
- Spreading diseases and parasites.
- Releasing reproductive materials of domesticated or non-native species that may contaminate the genetic integrity of local species[28].
- Slowing water flow, which may hinder sediment transport and alter marine chemistry.
- Competing for light with other marine organisms because of canopy shading by macroalgae.
- Competing for nutrients with other marine organisms, diverting nutrients away from wild food webs. This will be especially the case when massive seaweed cultivation is deployed to fulfil the global bioenergy demands[15].
- Causing environmental degradation through the construction of the farming system (e.g. destroying mangroves for wooden stakes or damaging the benthic ecosystem by clearing up the sea floor or using stakes or anchors).
- Causing pollution during operation.
- Growing seaweed and burying it in the deep ocean can help realize their full potential in carbon sequestration, but such operations face the challenges of economic viability and uncertain environmental impacts. In addition to nutrient competition and canopy shade, mineralization of submerged biomass creates a new oxygen demand on the seafloor and enriches the dissolved inorganic carbon concentration in the deep ocean. Effects are likely to be reversible, but could last a thousand years[29], see Blue carbon sequestration.
Seaweed harvesting can also entail adverse environmental impacts. An assessment of seaweed harvesting practices in Northern Island in areas of conservation importance, reported negative impacts related to disturbance of birds and wildlife, disruption of food webs, damage to substrata, habitat destruction, trampling, localised biodiversity changes, and changes in particle size distribution in sediments[23].
Measures for boosting seaweed ecosystem services
Market-based mechanisms, including carbon credits, nitrogen credits, blue bonds and green finance, among others, could be established to facilitate internalization of the positive externalities associated with seaweed cultivation[2], see Governance policies for a bio-based blue economy.
Related articles
- Kelp forests
- Diversity and classification of marine benthic algae
- Diverse applications of macroalgae
- Ecosystem services
- Governance policies for a bio-based blue economy
- Blue carbon sequestration
- Mariculture
References
- ↑ 1.0 1.1 1.2 1.3 1.4 Pereira, L. 2021. Macroalgae. Encyclopedia 1: 177–188
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 2.7 2.8 2.9 Cai, J., Lovatelli, A., Aguilar-Manjarrez, J., Cornish, L., Dabbadie, L., Desrochers, A., Diffey, S., Garrido Gamarro, E., Geehan, J., Hurtado, A., Lucente, D., Mair, G., Miao, W., Potin, P., Przybyla, C., Reantaso, M., Roubach, R., Tauati, M. and Yuan, X. 2021. Seaweeds and microalgae: an overview for unlocking their potential in global aquaculture development. FAO Fisheries and Aquaculture Circular No. 1229. Rome, FAO
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 Kraan, S. 2013. Mass-cultivation of carbohydrate rich macroalgae, a possible solution for sustainable biofuel production. Mitig. Adapt. Strateg. Glob. Change 18: 27–46
- ↑ Orfanidis, S. 2009. Temperature Responses and Distribution of Macroalgae Belonging to the warm-temperate Mediterranean-Atlantic Distribution Group. Botanica Marina 34: 541–552
- ↑ Charrier, B., Abreu, M.H., Araujo, R., Bruhn, A., Coates, J.C., De Clerck, O., Katsaros, C., Robaina, R.R. and Wichard, T. 2017. Furthering knowledge of seaweed growth and development to facilitate sustainable aquaculture. New Phytol. 216: 967–975
- ↑ Duarte, C.M., Gattuso, J.P., Hancke, K., Gundersen, H., Filbee-Dexter, K., Pedersen, M.F., Middelburg, J.J., Burrows, M.T., Krumhansl, K.A. and Wernberg, T. 2022. Global estimates of the extent and production of macroalgal forests. Glob. Ecol. Biogeogr. 31: 1422–1439
- ↑ 7.0 7.1 7.2 7.3 Froehlich, H.E., Afflerbach, J.C., Frazier, M. and Halpern, B.S. 2019. Blue Growth Potential to Mitigate Climate Change through Seaweed Offsetting. Current Biology 29: 3087–3093
- ↑ Smetacek, V. and Zingone, A. 2013. Green and golden seaweed tides on the rise. Nature 504, 84
- ↑ Dominguez, H. and Loret, E. 2019. Ulva lactuca, A Source of Troubles and Potential Riches. Marine drugs 17, 357
- ↑ Hengjie, T., Das, S.K., Zainee, N.F.A., Yana, R., Rozaimi, M. 2023. Ocean Acidification and Aquacultured Seaweeds: Progress and Knowledge Gaps. J. Mar. Sci. Eng. 11, 78
- ↑ 11.0 11.1 Bermejo, R., Buschmann, A., Capuzzo, E., Cottier-Cook, E., Fricke, A., Hernandez, I., Hofmann, L.C., Pereira, R. and van den Burg, S. 2022. State of knowledge regarding the potential of macroalgae cultivation in providing climate-related and other ecosystem services. Report prepared by an Eklipse Working Group
- ↑ 12.0 12.1 12.2 Biris-Dorhoi, E.S., Michiu, D., Pop, C.R., Rotar, A.M., Tofana, M., Pop, O.L., Socaci, S.A. and Farcas, A.C. 2020. Macroalgae—A Sustainable Source of Chemical Compounds with Biological Activities. Nutrients 12, 3085
- ↑ Abreu M, Pereira R, Sassi J-F. 2014. Marine Algae and the Global Food Industry. In: Pereira L, Neto J, eds. Marine algae, biodiversity, taxonomy, environmental impact assessment and biotechnology. Boca Raton, FL, USA: CRC Press, 300-319
- ↑ Hossain, M.S., Sharifuzzaman, S.M., Nobi, M.N., Chowdhury, M.S.N., Sarker, S., Alamgir, M., Uddin, S.A., Chowdhury, S.R., Rahman, M.M., Rahman, M.S., Sobhan, F. and Chowdhury, S. 2019. Seaweeds farming for sustainable development goals and blue economy in Bangladesh. Marine Policy 128: 104469
- ↑ 15.0 15.1 Kumar, M., Sun, Y., Rathour, R., Pandey, A., Thakur, I.S. and Tsang, D.C.W. 2020. Algae as potential feedstock for the production of biofuels and value-added products: opportunities and challenges. Sci. Total Environ. 716, 137116
- ↑ 16.0 16.1 Zang, K., Zang, F and Wu, Y.R. 2021. Emerging technologies for conversion of sustainable algal biomass into value-added products: A state-of-the-art review. Science of the Total Environment 784: 147024
- ↑ Raikova, S., Allen, M.J. and Chuck, C.J. 2019. Hydrothermal liquefaction of macroalgae for the production of renewable biofuels. Biofuels Bioprod. Biorefin. 13: 1483–1504
- ↑ 18.0 18.1 Ghallab, D.S., Ibrahim, R.S., Mohyeldin, M.M. and Shawky, E. 2024. Marine algae: A treasure trove of bioactive anti-inflammatory compounds. Marine Pollution Bulletin 199, 116023
- ↑ Kidgell, J.T., Magnusson, M., de Nys, R. and Glasson, C.R.K. 2019. Ulvan: a systematic review of extraction, composition and function. Algal Res. 39, 101422
- ↑ Zheng, Y., Jin, R., Zhang, X.,Wang, Q. and Wu, J. 2019. The considerable environmental benefits of seaweed aquaculture in China. Stoch. Environ. Res. Risk Assess. 33: 1203–1221
- ↑ Sangha, J.S., Kelloway, S., Critchley, A.T. and Prithiviraj, B. 2014. Seaweeds (Macroalgae) and Their Extracts as Contributors of Plant Productivity and Quality: The Current Status of Our Understanding. Advances in Botanical Research, Volume 71
- ↑ James, N.C. and Whitfield, AK. 2023. The role of macroalgae as nursery areas for fish species within coastal seascapes. Cambridge Prisms: Coastal Futures. 1, e3
- ↑ 23.0 23.1 McLaughlin, E., Kelly, J., Birkett, D., Maggs, C. and Dring, M. 2006. Assessment of the Effects of Commercial Seaweed Harvesting on Intertidal and Subtidal Ecology in Northern Ireland. Environment and Heritage Service Research and Development Series No. 06/26
- ↑ Duarte, C.M., Wu, J., Xiao, X., Bruhn, A. and Krause-Jensen, D. 2017. Can Seaweed Farming Play a Role in Climate Change Mitigation and Adaptation? Front. Mar. Sci. 4: 100
- ↑ Kim, J.K., Kraemer, G.P. and Yarish, C. 2014. Field scale evaluation of seaweed aquaculture as a nutrient bioextraction strategy in Long Island Sound and the Bronx River Estuary. Aquaculture 433: 148-156
- ↑ Barrington, K., Chopin, T. and Robinson, S. 2009. Integrated multi-trophic aquaculture (IMTA) in marine temperate waters. In D. Soto, ed. Integrated mariculture: a global review. FAO Fisheries and Aquaculture Technical Paper No. 529. Rome, FAO. pp. 7–46
- ↑ Hurd, C.L., Harrison, P.J., Bischof, K. and Lobban, C.S. 2014. Seaweed Ecology and Physiology, (2nd ed.). Cambridge University Press
- ↑ Schaffelke, B., Smith, J.E. and Hewitt, C.L. 2006. Introduced macroalgae – a growing concern. Journal of Applied Phycology 18: 529–541
- ↑ Wu, J., Keller, D. P., and Oschlies, A. 2022. Carbon Dioxide Removal via Macroalgae Open-ocean Mariculture and Sinking: An Earth System Modeling Study. Earth Syst. Dynam. 14: 185–221
Please note that others may also have edited the contents of this article.
|