Octylphenol
Definition of octylphenol:
The term octylphenol represents a large number of isomeric compounds. The octyl group may be branched in a variety of ways or be a straight chain and may be located at either the 2-, 3- or 4-position of the benzene ring. Of these potential isomers, 4-tertoctylphenol is the most commercially important[1].
This is the common definition for octylphenol, other definitions can be discussed in the article
|
Notes
| Octylphenol |
|---|
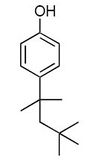
|
| Formula |
| C14H220 |
Octylphenol is in the EU produced (in 2001) at 23.000 tonnes per year. The main areas of use are as an intermediate in the production of phenol/formaldehyde resins and in the manufacture of octylphenol ethoxylates. These chemicals are used to in rubber, in pesticides and paints. Octylphenol mainly reaches the marine environment in waste waters from factories. Another possibility is from the breakdown of alkylphenol ethoxylates. It has also been reported that octylphenol is present as an impurity in nonylphenol and that this may account to some extent for its detection in the environment[1].
Considering the low vapour pressure of octylphenol and its tendency to adsorb to soils and sediments it can be expected that atmospheric concentrations will be low. Any octylphenol released to the atmosphere is likely to be degraded rapidly by reacting with hydroxyl radicals. In water and the soil however octylphenol is thought to be quite stable.
It has a low tendency to bioaccumulate and no tendency to biomagnify through food chains.
It is a toxic substance as concentrations above 6,1 µg/l can already have adverse effects on marine organisms.
The highest measured concentration (13 µg/l) is situated in the English Tees estuary. Sediment concentrations in the Tees estuary could reach levels up to 0,32 mg/kg dry weight. Concentrations in coastal water are usually below 0,016 µg/l. Concentrations in North Sea fish are bellow the limit of detection which is 0,004 mg/kg dry weight. In mammals and fishes it can cause endocrine disruptive effects, because it displays estrogen-like behaviour[1].
Environmental standards and legislation
Included in the OSPAR list of substances of priority action
Included in the water framework list of priority substances
See also
Octylphenol on the ED North Database
OSPAR background document on octylphenol
References
Please note that others may also have edited the contents of this article.
|