4-(dimethylbutylamino)diphenylamin
Definition of 4-(dimethylbutylamino)diphenylamine (6PPD):
high volumes of 4-(dimethylbutylamino)diphenylamine are used as a protective agent (anti-ozonant and antioxidant) in the rubber industry, mainly the tyre sector. Therefore, the main entry pathway of 6PPD to the environment is through tyre abrasion. At room temperature it appears as a brown solid[1].
This is the common definition for 4-(dimethylbutylamino)diphenylamine (6PPD), other definitions can be discussed in the article
|
Notes
| 6PPD |
|---|
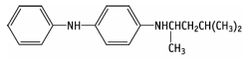
|
| Formula |
| C18H24N2 |
Although the main area of use of 6PPD is the tyre sector, the chemical is also found in other consumer products, such as seals of pressure cookers. Production in 2001 was estimated at 130.000 tonnes, of which 25.000 tonnes was produced in Europe (mainly Germany). 6PPD enters the environment mainly from the use and disposal of rubber products, but also through waste waters from manufacturing, cleaning and recycling. From the surface of rubber products, 6PPD might enter rivers with rain and despite its low volatility, it might evaporate to the atmosphere. Until the 1990s tyres were deposited in dumps, which resulted in mass 6PPD leakage. Since then, tyres are increasingly recycled or incinerated, which reduces 6PDD emissions[1].
6PPD is not a persistent substance. In the air, it is expected to be rapidly photodegraded (half-life of 1 hour) and in water, to be degraded by biotic and abiotic processes (half-life of 1 day). It has a low water solubility of 1 mg/l and because of its high tendency to adsorb to organic matter, 6PPD is only present in the water at very low concentrations. Although 6PPD is usually associated to sediments, no information is available on its stability in this medium. Therefore, it cant be ruled out that 6PPD persists and accumulates in the sediments. Due to the low stability of 6PPD in aqueous media, it's expected, despite its high affinity for organic matter, unlikely to bioaccumulate in wild organisms or to biomagnify through food chains.
Acute toxicity is caused by both 6PPD and, at a lower level, by its degradation products. 6PPD concentrations of 0,028 mg/l is acutely toxic for the most sensitive fish species. Zooplankton can experience effects of 6PPD at concentrations above 0,05 mg/l, and high mortality at concentrations above 0,5 mg/l. To rats 6DPP becomes acutely toxic when administered at doses above 3,58 g 6PPD per kg body weight[2].
Since exposure of 6PPD to the environment and transport to the sea is anticipated to be low, risks to marine organisms are expected to be negligible. Nevertheless, 6PPD is a high production volume chemical. This justifies a continuous attention towards this substance and its metabolites[1].
Environmental standards and legislation
Included in the OSPAR list of substances of priority action
See also
OSPAR background document on 6PPD
References
Please note that others may also have edited the contents of this article.
|