Difference between revisions of "Nature-based shore protection"
Dronkers J (talk | contribs) |
Dronkers J (talk | contribs) |
||
| Line 6: | Line 6: | ||
Beaches and dunes can also be considered living shorelines. The article [[Shore protection vegetation]] gives and introduction to the role of vegetation for the protection of dune coasts. Shore nourishment is a widely practiced nature-based coastal protection measure for beach and dune coasts worldwide, see [[Shore nourishment]] and further references therein. | Beaches and dunes can also be considered living shorelines. The article [[Shore protection vegetation]] gives and introduction to the role of vegetation for the protection of dune coasts. Shore nourishment is a widely practiced nature-based coastal protection measure for beach and dune coasts worldwide, see [[Shore nourishment]] and further references therein. | ||
| − | Articles dealing with so-called '''soft coastal protection measures''' are listed in the category [http://www.coastalwiki.org/wiki/Category: | + | Articles dealing with so-called '''soft coastal protection measures''' are listed in the category [http://www.coastalwiki.org/wiki/Category:Soft_coastal_interventions Soft coastal interventions]. |
Revision as of 16:57, 19 September 2023
Nature can offer shore protection in addition to or instead of manmade hard structures. Nature-based shore protection is provided by living shorelines that generate in addition many other ecosystem services. In this article, under the general term 'living shorelines' the following will be included: (a) salt marshes, (b) benthic vegetation such as seagrasses, (c) biogenic reefs.
This article focuses on nature-based shore protection in temperate climate zones. Mangroves are dealt with in the article Mangroves and coral reefs in the article Coral reefs.
Beaches and dunes can also be considered living shorelines. The article Shore protection vegetation gives and introduction to the role of vegetation for the protection of dune coasts. Shore nourishment is a widely practiced nature-based coastal protection measure for beach and dune coasts worldwide, see Shore nourishment and further references therein.
Articles dealing with so-called soft coastal protection measures are listed in the category Soft coastal interventions.
Contents
Introduction
Narayan et al. 2016[1] reviewed the functionality of natural barriers as nature-based coastal defence structures. Their analyses and syntheses demonstrated the following: a) coastal habitats–particularly coral reefs and salt-marshes–have significant potential for reducing wave heights and providing protection at the shoreline; b) restoration projects for which data are available–i.e., mangrove and marsh projects–can be cost-effective relative to submerged breakwaters in attenuating low waves and become more cost-effective at higher water depths; c) a number of nature-based defence projects, especially in mangroves and marshes, have been observed to offer protection during storms. Variations in wave reduction and cost effectiveness are dependent on multiple parameters including water depth and vegetation / reef height.
Van Coppenolle and Temmerman 2019[2] investigated the potential of natural barriers as nature-based protection structures for 135 coastal cities worldwide. Their study reveals that 60% (8 300 km2) of the [city]-area below mean high tide is urbanized or densely populated and 34% (4 600 km2, distributed over 124 cities) is potentially available for tidal wetlands restoration or creation. Key factors influencing this potentially available space are the geomorphology as well as the population density in the coastal area in front of the city. The land use in the potentially available area for tidal wetlands restoration or creation is mainly composed of croplands, paddy fields, water bodies and vegetated areas, and influences the effectiveness of tidal wetland creation for nature-based flood risk mitigation.
Seagrasses
General features
Seagrasses are dioecious marine angiosperms which have evolved from terrestrial ancestors. They produce flowers, fruits and seeds and are typically secured to silt or sandy sediment by a matrix of roots and rhizomes where meadow density can range from hundreds to thousands of shoots per m2 (Milchakova & Phillips, 2003 [3]). They occur globally, in shallow waters with water depth less than 90m (Duarte, 1991[4]) and consist of approximately 50 species (den Hartog, 1977[5]), with an estimated global coverage of over 177,000 km2. Many seagrass species are similar in appearance, having monopodial growth of long straplike leaves supported by sediment stabilising roots and rhizomes and can exist in fully marine through to fresh water habitats. Most seagrasses colonize soft substrates such as sand in quiescent areas (i.e., wave-sheltered) (Koch et al., 2006[6]); however, some seagrasses, such as those of the genus Phyllospadix and sometimes Posidonia attach to rocks and are exposed to relatively high wave energy. Of about 50 species worldwide, only seven seagrass species are native of European waters. In spite of their terrestrial origin, seagrasses are well adapted to the marine environment and can in Europe be found from the intertidal zone at the shore to depths down to 50-60 m. The European species are easy to identify and their geographical distribution range is well known. The main four European species of seagrasses are: (a) Zostera marina (eelgrass), (b) Zostera noltii (dwarf eelgrass), (c) Posidonia oceanica and (d) Cymodocea nodosa (Borum et al., 2004[7]). See the article Seagrass meadows for a more complete introduction to seagrass ecosystems.
Species and Characteristics
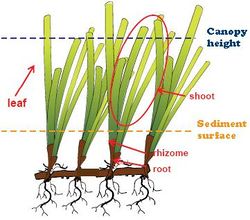
Seagrasses have a horizontal rhizome linking clusters of leaves referred to as shoots or stems, and roots are usually found at each shoot/stem as shown in Figure 2 (Koch et al., 2006 [6]). When most of the plant biomass occupies a large portion of the water column, the vegetation is often referred to as a canopy. In contrast, when most of the seagrass biomass is found near the bottom, they are called meadows. The characteristic parameters which can affect the wave propagation and dissipation over a seagrass canopy are: (a) the seagrass density, (b) the canopy height expressed with the submergence ratio (canopy height over the water depth) and (c) the bending of the shoots. The amount of seagrass present is quite often quantified by counting the number of stems in an area (e.g., 25 x 25 cm) and extrapolate that to stems per m2. This parameter called “seagrass density” varies between species. A dense Ruppia maritima bed may have more than 3,000 stems/m2 while a dense Zostera marina bed may only have 2,000 stems/m2 and Posidonia oceanica has about 400 stems/m2 (Koch et al., 2006[6]). Canopy height is also an important seagrass parameter when evaluating the impact of seagrasses on water flow. This parameter is usually obtained by averaging the tallest two-thirds of the leaves. The smallest seagrasses (genus Halophila) are only 2 or 3 cm tall while the largest seagrasses can reach 2 m in length (nonreproductive Zostera marina and Phyllospadix). Bending of the shoots influences the flow under wave action as well (Bradley and Hauser, 2009[8]).
Coastal protection function
Wave attenuation
The system is characterized by complex hydrodynamics since seagrasses and water flow may interact in highly coupled, nonlinear ways (Koch et al., 2006[6]). This interaction is dynamic since the structure of aquatic plant fields changes with time and is exposed to variable physical forcing of the water flow (Mendez and Losada, 2004[9]). The function of these natural barriers as coastal protection systems can be summarised to the following functions: wave attenuation, protection of the hinterland from flooding and stabilizing the seabed. The degree of wave attenuation depends both on the seagrasses characteristics (the seagrass density, the canopy height, the stiffness of the plant and the bending of the shoots) and the wave parameters (wave height, period and direction) so the quantification of wave energy dissipation over seagrasses is difficult to express in a simple way. Various studies on wave attenuation by coastal vegetation have been performed (e.g. Fonseca and Cahalan, 1992[10]; Bouma et al., 2005[11]; Fernando et al., 2008[12]; Stratigaki et al., 2010[13]) where the results show differences in the degree of wave attenuation.
Protection against flooding by storm surges
Flooding is usually associated with severe storm events. Therefore, it could be speculated that seagrass beds will be less effective in attenuating waves (canopy occupies a smaller fraction of the water column) when this ecosystem service is needed the most. However, storms also generate longer waves. Therefore, theoretically, seagrasses may still attenuate waves during storm events when flooding occurs (Koch et al., 2006[6]). An ecosystem service commonly listed for seagrasses is sediment and shoreline stabilization[14]. Due to their capacity to alter their environment, seagrasses have been referred to as ecosystem engineers, because they partly create their own habitat. This is achieved by slowing water motion and current flow and by reducing sediment suspension, whilst directly influencing sediment composition through accretion of biological particles from the seagrass itself and by retaining fragments of shell and skeletons of the numerous organism that inhabit the meadow (Fonseca and Cahalan, 1992[10]; Borum et al, 2004[15]). However, field observations by Oprandi et al. (2020[16]) on the Ligurian coast (NW Mediterranean) showed great destruction of seagrass meadows by a severe storm in 2018, reducing the Posidonia oceanica cover by 50%.
Performance and sensitivity
Ward et al. (1984[17]) performed field measurements in a shallow estuarine embayment colonized by seagrass communities in Chesapeake Bay, USA, focusing on the effect of seagrasses on wave attenuation and on suspended material. They showed that wave energy was attenuated by the vegetation, suppressing resuspension and enhancing sedimentation rates. The interaction of flow and seagrass canopies of Amphibolis antarctica species, which differ morphologically from more commonly studied blade-like seagrasses such as Zostera and Thalassia is described in Verduin and Backhaus (2000[18]). A series of velocity measurements were obtained within, above and adjacent to A. antarctica meadows at different heights above the seabed for swell wave conditions of the study area (T=13-16.5 s). The effect of seagrass canopy on flow was shown to be an overall damping effect. Granata et al. (2001 [19]) measured the particle and flow distribution within seagrass meadows in a Northeast coast of Spain for both low and high wave and current activity. For the low-energy period, the vertical reduction of the total kinetic energy was larger in the meadow than over the sand. Moreover results show the 3-dimensionality of the meadow, since the meadow acts as a bluff body diverting flow over the meadow, which is the path of least resistance, while creating a secondary circulation cell at the meadow’s edge thus producing complex, 3-D transport patterns. Tigny et al. (2007[20]) conclude that P. oceanica meadows in the Gulf of Oristano (west coast of Sardinia, Italy) significantly affect the littoral geomorphology, providing biogenic sediments, controlling beach slope, and acting as a ‘‘brake’’ on coastal water masses. Brandley and Hauser (2009[21]) performed a field study in a microtidal bay in northwest Florida, where the main species of the meadows were Thalassia testudinum and Halodule wrightii. The purpose of the study was to quantify the attenuation of incident wave height through a seagrass meadow and characterize the blade movement under oscillatory flow under the low-energy conditions characteristic of fetch-limited and sheltered environments. They found that the resulting wave height decay for submerged vegetation is described as an exponential function, with an average decrease of the significant wave height of 30% for a distance of 39 m. They also found that the ability of the seagrass to attenuate wave energy decreases as incident wave heights increase and conditions become more turbulent. A review of the coastal protection function of seagrass meadows by Ondiviela et al. 2014([22]) concluded: " Seagrass meadows cannot protect shorelines in every location and/or scenario. The efficiency of the protection depends largely on the incident energy flux, density, standing biomass and plant stiffness. The optimal conditions for enhancing the defense provided might be achieved at shallow waters and low-wave energy environments, with high interaction surface, in the vertical and horizontal dimension, between water flow and seagrasses, but less so when hydrodynamic conditions are more severe. Likewise, the most favorable protection might be offered by large, long living and slow-growing seagrass species, with biomass being largely independent of seasonal fluctuations and with the maximum standing biomass reached under the highest hydrodynamic forcing".
Sea level rise
The effect of sea level rise on seagrasses should be seen in the frame of the effect of global climate change and rise in water temperature. The primary effect of increased global temperature on seagrasses will be the alteration of growth rates and other physiological functions of the plants themselves. The distribution of seagrasses will shift as a result of increased temperature stress and changes in the patterns of sexual reproduction (Short and Neckles, 1999 [23]). Seagrass distribution shifts could be even greater if oceanic circulation changes in response to global warming, leading to abrupt changes in water temperature beyond those directly resulting from warming, as water masses shift at the edge of present biogeographical boundaries between seagrass floras (Duarte; in Borum et al., 2004). The rise in sea level may have numerous implications for circulation, tidal amplitude, current and salinity regimes, coastal erosion and water turbidity, each of which could have major negative impacts on local seagrass performance (Duarte[24]; in Borum et al., 2004[25]). The greatest direct impact of an increase in sea level will be an increase in the depth of water and the consequent restricting available light to the benthic vegetation (Koch and Beer, 1996[26]). A 50-centimeter increase in water depth due to sea level rise may result in a 30-40% reduction in seagrass growth (Short and Neckles, 1999[23]). On the positive side, increases in current velocity within limits may cause increases in plant productivity reflected in leaf biomass, leaf width, and canopy height (Bjork et al. 2008 [27]). The overall effect of sea level rise would result in less efficient coastal protection function. Since the degree of wave attenuation and flooding mitigation depends on the fraction of water column occupied by the canopy, an increase on the sea level rise would result in smaller fraction and thus less protection. Subsequently the leaves would be less effective in trapping suspended material and sedimentation would decrease resulting in decrease of coastal zone protection against erosion.
Salt marshes
General features
A salt marsh is an environment in the upper coastal intertidal zone between land and salty or brackish water, dominated by dense stands of halophytic (salt-tolerant) plants such as herbs, grasses or low shrubs (Adam, 1990[28]); an aerial view of a salt marsh is shown in Figure 3. The lowlands are protected from marine flooding by salt marshes, which provide through their vegetation a means of damping storm waves (Allen, 2000[29]). Salt marshes are coastal ecosystems that are being influenced by waves and tide. They develop favorably on gently sloping shores with little wave energy and sufficient sediment supply (Janssen-Stelder, 2000[30]; Dijkema, 1987[31]). Therefore salt marshes can usually be found in the sheltered areas behind offshore barrier islands, behind spits, in estuaries and in protected bays with shallow water (Chapman, 1976[32]).
Seven types of marsh (Figure 4) are recognized by Allen (2000[33]) and Pye and French (1993[34]). Open-coast marshes typically are sandy systems coupled with relatively exposed sandflats (Fig. 4a). Open-coast back-barrier marshes are sandy-muddy systems found on the sheltered, landward sides of coastal barrier islands and spits (Fig. 4b). Open-embayment marshes fringe the edges of large tidal embayments with unobstructed entrances and tend to be sandy (Fig. 4c). Rivers may drain into such embayments. The sandy-muddy, restricted-entrance embayment with marshes is partly closed off at the mouth by one or more spits or promontories (Fig. 4d). Marshes within estuaries range between the fringing and back-barrier types, depending on the degree of obstruction of the estuary mouth (Fig. 4e and f). The flora of a salt marsh is differentiated into levels according to the plants' individual tolerance of salinity and water levels. The most common salt marsh plants are glassworts (Salicornia spp.) and the cordgrass (Spartina spp.), which have worldwide distribution. See the article Salt marshes for a more complete introduction to salt marsh ecosystems.
Coastal protection function
Wave attenuation
Salt marshes can in two ways contribute to enhancing the effectiveness of coastal defenses: i) in wave exposed areas, marsh vegetation can be capable of dissipating over 90% of incident wave energy over tens of meters (Möller, 2006[35]) and ii) in more sheltered up-stream estuarine areas, the marsh offers water storage volume during spring tides or high river discharge. A study on the Norfolk coast (UK) by Möller et al. (1999[36]) showed that wave energy dissipation rates over the salt marsh were significantly higher (at an average of 82%) than over the bare sand flat (at an average of 29%). The average reduction of wave heights over 200 m was found to be about 63%. Numerical modelling confirmed friction by vegetation to be the main reason for wave attenuation. An important aspect in wave attenuation is that the root system of the marsh vegetation stabilizes the sediment against wave attacks. The efficiency of hydrodynamic attenuation varies with plant community structure. Tall and rigid vegetation are more efficient dissipating wave energy than short and flexible species (Bouma et al., 2005[37]), but this also depends in a complex way on the density of vegetation canopy. However, according to a study by Möller (2006[35]), vegetation type/density has no significant direct effect on wave attenuation, but modifies the process of wave transformation. He found that wave attenuation increases with wave height, but remains approximately constant the ratio wave height/water depth is greater than 0.55. Figueroa-Alfaro et al. (2022[38]) showed that estimates of vegetation-induced friction can be derived at low cost from satellite imagery of marsh vegetation.
Protection against flooding by storm surges
A model study of marsh reclamation on the Shanghai coast showed that for every km of tidal flat ranging from high marsh to bare tidal flat that is being regained, the sea dike can be lowered by 0.84 - 0.67 m, when designing for a 1 in 200 years storm event[39]. However, the vegetation protection is less effective in situations where the stems of marsh plants are broken by very high waves[40]. The reduction in water level per km depends not only on vegetation type but also on storm surge duration. In a surge steadily rising by 2m over 1 h, the reduction in water level over a saltmarsh can amount to about 45 cm/km but the reduction is approximately halved if the surge rise takes 2 hours[41].
Storm surge levels far above the high tide level can occur on coasts which are situated on wide shallow continental shelves. Salt marshes situated in front of the dike offer less protection in this case. Artificial high zones and breakwaters on the salt marsh improves the flood defense's reliability, against relatively low costs compared to dike heightening[42][43].
Performance and sensitivity
Effect of grazing
The marsh vegetation has a key importance to coastal protection through consolidation of the soil and by representing a structural hindrance to wash-over waves. Livestock has large potential for altering vegetation structure directly through feeding and indirectly by altering the environmental conditions. Therefore, grazing management probably holds relevance to the protective capacity of marshes although there has been little quantitative research on this subject. Feeding and defecation moderates vegetation structure-composition and above- and below-ground biomass production while trampling and hoof holes may lead to soil compaction and can cause saltpan formation. Intense grazing modifies zonation patterns and transforms complex woody shrub communities into homogenous lawns dominated by short flexible grass, with an associated likely reduction in wave attenuation and sedimentation rates. Grazing at low intensity increases vegetation patchiness which may cause specific spatial patterns in turbulence and sedimentation, so that the sum effect of patchiness on marsh coastal protection is not known. Conversely, grazing pressure can lead to greater resource allocation to below-ground biomass, thus reducing surface erosion and below ground contributions to an increase in marsh surface elevation.
Marsh erosion
Loss of salt-marsh habitat due to lateral erosion is a major problem across Europe. For example, the estuaries of south-east England lose about 40 ha yr-1 of tidal-marsh area due to lateral erosion (Hughes & Paramor, 2004[44]). Tidal-marshes can erode by (1) lateral erosion of the exposed marsh-edge, and (2) channel migration. Lateral erosion can set in when the seaward edge of the tidal salt-marsh becomes disturbed (cliff-erosion). Wave disturbance, for example a storm surge or waves by shipping traffic, can initialize this erosion process by forming a steep slope. This cliff is more vulnerable to wave action so that the erosion is not easily halted. Similarly, erosion of riparian vegetation along creeks dissecting the marsh can take place resulting in the widening of the channels. It is generally believed that human activities and disturbances greatly increase the rates at which erosion occurs. Pollution, shipping traffic, and dredging activities are considered to have substantial effect on erosion rates. However, natural causes of erosion are also known. Biotic disturbances of the marsh vegetation are for example the worm Nereis and crab which both affect the roots by burrowing. Intrinsic dynamics can cause marshes to become vulnerable to erosion as well. Due to the vegetation-sediment feedback the seaward salt-marsh edge becomes steeper in time allowing subsequent cliff erosion. However, human influences can have indirect effects on the severity of these biotic treats.
Managed realignment
The current effort to restore marsh systems in Europe and elsewhere represents evidence of the political and managerial value placed on the goods and services provided by this ecosystem. The principle of 'managed realignment' and 'managed retreat' is one of allowing salt-marsh areas that were historically converted to alternative use for anthropogenic purposes (e.g. agricultural land or tourist development) to return to their natural state and area cover. This can be done in a number of ways, but typically involves making a breach in the historically erected barrier (seawall, dike) rather than removing the whole structure. This approach reduces the cost involved, as well as the wave action depressing the development of the vegetation. Low-lying inland areas must be protected from flooding with a dike, because high water levels are not significantly reduced and in some cases even amplified in the recovered salt marsh[45].
Experience of estuarine restoration by managed realignment projects during the past decades has shown that the estuarine character of newly created mudflats and marshes is easily lost (Mazik et al., 2010[46]). Main problems that emerge are (a) rapid sedimentation, so that tidal flooding only occurs exceptionally, and (b) marshes reactivated by managed realignment do not provide habitats and species in comparable proportions to natural marshes and do not have equivalent biological characteristics (Mossman et al., 2012[47]). Marsh accretion is very fast in dynamic turbid estuaries, but occurs at longer term in any estuary and therefore requires active management involving, e.g. bed leveling, dredging and flushing (Pontee, 2014[48]).
Sea level rise
Coastal squeeze is a serious problem for marshes to cope with sea-level rise. The coastal defense value of salt-marshes can be severely compromised by lateral marsh erosion, as a sufficient width of marsh perpendicular to the sea is required to significantly reduce wave energy. Acknowledgements of such area dynamics for several wetland systems have given rise to important recent policy, such as 'making space for water' in the UK. However, much is still unclear about the effects of marsh vegetation traits, as well as the importance and interactions between environmental parameters, such as relative exposure, sediment load in water, nutrient input, and tidal volume. Management decision and the consequences for the coastal defense-value of marshes is therefore currently not always based on sound scientific knowledge. For more detailed information, see Dynamics, threats and management of salt marshes.
Marsh vegetation plays a key role in raising the marsh surface by trapping sediment. However, a serious risk exists that rising relative sea levels result in drowning of salt-marshes because accretion is not able to keep pace with sea level rise[49]. Erosion at the creek bank can reduce the outer limit of the marsh area. If the up-shore migration of the marsh is then also physically blocked by a dike or seawall the marsh might be squeezed between the rising sea level and the fixed barrier. This principle of 'coastal squeeze' represents a significant risk to many marshes worldwide (Kirwan et al. 2016[50]). It is highly probable that along with sea level rise, the frequency of stormy events will increase, with greater marsh erosion as a possible consequence. However, the effects of changing climate conditions on salt-marshes are still poorly understood.
Biogenic reefs
General features
The classic definition of a reef is a submerged structure rising from the surrounding seafloor that forms a hazard to shipping (Wood, 1999[51]). In recent years, this definition has undergone several transformations due to emerging exceptions to this rule (e.g. cold-water coral reefs, Roberts et al., 2006[52]). Modern
consensus now defines reefs as being any structure in the marine environment that arises from the seabed and covers an extensive area. Some management agencies have expanded their interpretation of reef to include both, geogenic formations of bedrock, cobbles or boulders and biogenic concretions created by structure-forming coral, bivalve and polychaete species. It must be noted, that there is no uniform structure to reefs, they vary in scale and extent and the life they support is greatly dependent upon its location and composition. In the waters around Europe, several key organisms form biogenic reefs. These can range from enormous structures formed by cold-water coral species such as Lophelia pertusa (Roberts et al., 2009[53]), to smaller aggregations of tube-building polychaete worms such as Sabellaria spinulosa (Holt et al., 1998[54]). Reefs formed by scleractinian cold-water corals such as Lophelia pertusa occur at great water depths, typically between 200-1000 m. Therefore, they do not play a role for coastal protection.
Species and Characteristics
In this section, besides S. spinulosa, aggregations of the bivalve Mytilus spp., the polychaetes Sabellaria alveolata and Serpula vermicularis will be reviewed. See also the article Biogenic reefs of Europe and temporal variability.
Sabellaria spinulosa
In contrast to S. alveolata, the closely related Sabellaria spinulosa is typically recorded sub-tidally and only rarely in intertidal habitat (Foster-Smith & Hendrick, 2003[55]). This species is common in solitary or small aggregations, but under favourable conditions can be gregarious, developing into thin layers and large reef-structures that can be up to around 30 cm high (Hendrick & Foster-Smith, 2006[56]). The sedimentary composition of tubes show similarities with S. alveolata, but morphologically are much thinner and upright. The tubes, whilst fragile, are deceptively strong and are formed by several layers of sediment ranging from large particles on the exterior to smaller particles on the interior with a parchment-like interior tube.
Sabellaria alveolata
Sabellaria alveolata is a sedentary tube-dwelling polychaete that constructs tubes from suspended sediment and shell fragments (Wilson, 1971[57]). Although S. alveolata does occasionally occur as individuals, it is more commonly found in colonies. The colonies form bio-constructions which typically come in two major forms: Veneers, which closely adhere to rocks and are very common at the mid shore of the intertidal zone and can be up to 30cm in height (Wilson, 1971 [57]). Reefs, which are generally found at the lower level of the intertidal zone, are up to 1.5 m in height and can develop to cover acres of sand flats (Gruet, 1982[58]). This species is typically located on exposed, open coasts with reasonable to substantial water movement (Cunningham et al., 1984[59]). It is predominantly intertidal, but has been observed sub-tidally on rare occasions (e.g. the Severn Estuary, Mettam, 1994[60], off the Cumbrian coast, Perkins, 1980[61]). The tubes of S. alveolata in dense aggregations form a characteristic honeycomb structure, tubes are narrow at the base, but widen to create a porch-like opening at the entrance. This structure is easily damaged, and undergoes almost continual repair by living worms (Wilson, 1971[57]). The records of Sabellaria alveolata throughout Europe are greater in northern latitudes. This is an obvious artefact of data reporting to OBIS as S. alveolata has been reported to be widely distributed in the France, Spain and Portugal and extends as far south as Morocco (Gruet, 1982[58]; Cunningham et al., 1984[59]). This species builds the largest reefs on the European coast; in particular the “Les Hermelles” reef in the Baie du Mont Saint Michel in France is over 100 hectares (Figure 6) and is considered the largest reef in Europe (Gruet, 1982[58]; Marchand & Cazoulat, 2003[62]).
Serpula vermicularis
The serpulid worm Serpula vermicularis builds calcareous tubes that can be 4-5 mm in diameter and 150mm in length. The tubes are occasionally ringed and cylindrical in form, with lengthwise ridges. This species is usually solitary, but aggregations that can be classed as reefs have been recorded at sheltered locations on the west coast of Scotland (Poloczanska et al., 2004[63]; Dodd et al., 2009[64]). Their tubes are attached to hard substrata, sub-tidally to depths of a maximum of 250 m. Reefs formed by this species are particularly rare and occur only in well sheltered sea lochs with limited currents and wave exposure.
Mytilus spp.
Mytilus edulis and Mytilus galloprovincialis co-occur throughout much of Europe. Despite being two distinct species, they are difficult to identify in the field and are known to hybridize. In this review, we simply refer to both species together at Mytilus spp. Aggregations of Mytilus spp. are found in shallow subtidal along much of the coast of Europe. This species has a major reef-forming role, as Mytilus spp. can form bio-constructions that range in size from small clumps to beds of several hectares (Dankers et al., 2001[65]). Individuals of this species are usually semi-infaunal, projecting above the sediment, creating an irregular surface topography (Commito and Rusignuolo, 2000[66]). This surface complexity alters water movement over the bottom, producing boundary layer flow regimes that affect the delivery and resuspension of sediment particles in mussel beds (reviewed in Commito and Dankers 2001[67]; Widdows and Brinsley, 2002[68]; Widdows et al., 2002[69]; Gutiérrez et al., 2003[70]). In addition to structuring the hydrodynamic environment, Mytilus spp. also modify their environment by actively filtering sediment and the subsequent formation of biodeposits (Flemming and Delafontaine, 1994[71]; Oost, 1995[72]). The heterogeneous topography generated by the mussels also consolidate and entrain sediments (Widdows and Brinsley, 2002[68]).
Coastal protection function
Reefs have a major functioning role in European coastal and deep seas, providing a range of goods and services such as storm protection and flood control. In a significant study, Costanza et al (1997[73]) valued 17 ecosystem services offered by habitats throughout the world, particularly pertinent for reefs are disturbance regulation, erosion control and sediment retention, nutrient cycling, refugia and recreation. By extrapolating these services to economics, a value of $6,075 ha-1 yr-1 was attributed to tropical coral reefs. The authors estimated that 45% ($2,750 ha-1 yr-1) of this value could be attributed to disturbance regulation in the form of sea-defence. Whilst tropical reefs are obviously exceptional in European seas, the same relative importance can be attributed to reefs in other habitats. Ecosystem goods and services can be broadly defined into several key themes, regulation, habitat, production and information (De Groot et al., 2002). Particularly pertinent for natural reefs is regulation. This service is provided in a range of forms, principally, regulation against disturbance, biofiltration improving water quality, sediment retention and the byproduct of structural habitat, biodiversity. Disturbance regulation relates to the ability of reefs to mitigate natural hazards and events. These hazards and events can include breaking waves, storms and flooding. The effects of these are becoming increasingly costly, as they intensify under environmental change (De Groot et al., 2002[74]). There is no doubt that reefs have great value in attenuating the effects of these events. Tropical reefs have been long described as an example of how natural structures can protect coastlines from damage due to wave energy (Moberg & Rönnbäck, 2003[75]). However, the contribution of temperate reefs around Europe towards regulating these disturbances has been largely understudied. In addition to disturbance regulation, many reefs also directly and indirectly enhance the consolidation of sediments through the presence of their structures. Reef frameworks also serve to entrap mobile sediments, often increasing local sediment loading and sometimes contributing to the development of the reef itself. Perhaps the greatest service provided by reefs is the provision of structural habitat that generally increases habitat heterogeneity. This results in the creation of space and ultimately, niches that can be exploited by other species.
Performance and sensitivity
Biogenic reef builders (including mussel beds and worm reefs) have recently been named ”bioconstructors” (Bianchi, 2001[76]). They can be defined as organisms that build films, crusts, mounds or reefs of material that they either produce internally (e.g., biogenic carbonate deposition), bind from other sources (using organic cement e.g. worm reefs) or develop from a combination of the two (e.g. mussel beds). The majority of research on biogenic reefs has focused on the morphology of the structures and their function in enhancing biodiversity by habitat creation (e.g. Dubois et al., 2002[77]; O’Connor and Crowe, 2007 [78]). Few studies have focused specifically on the geomorphological contributions of bioconstructions themselves, particularly in relation to their potential roles in natural coastal defence. Hence, there is much need for research on natural reefs both in terms of their intrinsic functions such as growth, development, resistance and resilience as well as their importance in larger scale ecosystem functioning which may benefit developing predictive models of biocomplex responses to predicted sea-level rise and global climate change.
Sabellaria spinulosa usually occurs subtidally in areas of high water flow, and is relatively tolerant of wave and tidal-forcing. However, it has been suggested that an increase in wave or tidal flow may reduce the stability of the attachment substratum resulting in increased scouring and mortality of individuals (Jackson & Hiscock, 2008[79]). It is a relatively disturbance-tolerant pioneers species (Jackson & Hiscock, 2008 [79]). Fisheries for the pink shrimp Pandalus montagui and brown shrimps Crangon crangon have been implicated in the loss or damage of reefs but experimental and observational studies have indicated only minor damage to tubes and rapid recovery as a result of shrimp fisheries. Nevertheless, populations, especially if as loose aggregations, may be displaced by mobile fishing gear. In gregarious aggregations, Sabellaria spinulosa tubes intertwine to form a rigid structure which collects sand, detritus, and fecal material between the tubes (Hendrick & Foster-Smith, 2006[80]). In reefs, the bound sediment comprising the S. spinulosa tubes typically smothers the underlying substrate. In contrast, the consolidated tubes of crustose colonies typically form a thin veneer across the surface of the underlying substratum and do not necessarily cement larger pebbles together. Their ability to trap sediment is less and this growth form is considered to be more susceptible to fragmentation during winter storms for instance and to damage from physical impacts than a more upright morphology (Holt et al., 1998 [54]). Davies et al. (2009[81]) found that S. spinulosa consistently utilised a lower mean particle size than that of the background sediment when provided with well sorted medium sands. Under sediment starved conditions, there was net erosion of colonies whereas under intermediate and high sediment rates there was consistent cumulative growth. This means S. spinulosa requires water to be relatively turbid (i.e. high flow periods and storm events) in order for the tubes to grow. A crucial characteristic when considered as a coastal defense mechanism.
Sabellaria alveolata contructs tubes from sand-sized mineral grains and shell debris, referentially sorting sand grains and accumulating heavy minerals (Fager, 1964[82]; Gram, 1968[83]; Multer and Milliman, 1967[84]). S. alveolata is widely distributed throughout Europe. It forms veneers and reefs. Reefs are generally found at the lower level of the intertidal zone (Gruet, 1982 [58]) and can be up to 1.5m in height. Thus, the reefs perform indirect and direct bioprotectional roles as they physically build structures that might influence local hydrodynamics or energy regimes, and preferentially store sediment that would otherwise be “loose” in the system, available to physically abrade shore platforms (Naylor and Viles, 2000 [85]; Naylor, 2001 [86]). Sabellaria alveolata is typically threatened by the physical disturbance of removal from tubes and substratum loss. It has been suggested that most colonies die through eventual break up by wave action (Jackson, 2008[79]). Increased exposure will potentially result in shorter colony lives. S. alveolata is a southern species and is at the northern end of its range in Britain. It has been shown to be severely affected by low winter temperatures (Crisp, 1964[87]). Despite the current trends in global warming, winter 2009/2010 was the coldest on record in Europe, which may have negatively affected S. alveolata at its range edges. Continued monitoring is necessary to detect future changes.
S. vermicularis is a subtidal species found permanently attached to the substratum. It is relatively intolerant to strong increased water flow rates and strong wave action which interfere with its feeding. If strong wave action occurs over prolonged periods of time, death can occur. S. vermicularis forms reefs in sheltered areas (e.g. sea lochs) where it is likely to be even more intolerant to wave action.
Large aggregations of Mytilus spp. are found in shallow marine environments along much of the European coast of Europe. Mytilus spp. is a major bioconstructor, forming rough and sediment retaining mussel patches that range in size from small clumps to large beds of several hectares (Dankers et al., 2001[65]). Mytilus spp. also modify their environment through active filtration and the subsequent formation of biodeposits of seston (Flemming and Delafontaine, 1994 [71]). Mussels could be used to reduce turbidity by biofiltration (Beukema and Cadée, 1996[88]), which may benefit the reintroduction of sea grasses (Van Katwijk, 2003[89]). The beds can dissipate wave energy, thereby protecting salt marshes from erosion (De Vries et al., 2007[90]). Extra deposition of fine sediments in these areas by a reduction of flow velocities or fixation as (pseudo-) fecal matter is also thought to increase the resilience of salt marshes (Van Leeuwen et al., 2010). Mytilus spp. is semi-infaunal. Projecting above the sediment, individuals create an irregular surface topography (Commito and Rusignuolo, 2000) which produces a boundary layer flow over the bottom that affects the delivery and resuspension of sediment particles in mussel beds (reviewed in Commito and Dankers, 2001; Widdows and Brinsley, 2002[68]; Widdows et al., 2002[69]; Gutierrez et al., 2003). In a recent study, Van Leeuwen et al (2010[91]) simulated a process-based model of the interaction between a young mussel bed and fine sediment. It was concluded that a combination of active deposition via filtration and slow down of the flow due to increased roughness leads to high net deposition in the mussel bed. In addition, young mussels can quickly climb on top of deposited material resulting in rapid trapping of large amounts of fine sediment. In the wake of the mussel bed, deposition is also high because of reduced flow velocities. Repeated substratum loss and recruitment results in a patchy distribution of mussels on the shore (Seed & Suchanek, 1992[92]). Storms and tidal surges are known to destroy mussel beds, often over hundreds of hectares in the Wash, Morecambe Bay and the Wadden Sea. Feasibility of using the biogeomorphological impact of mussel beds for ecological engineering purposes (Odum and Odum, 2003[93]) is currently under investigation.
Related articles
- Biogenic reefs of Europe and temporal variability
- Dynamics, threats and management of biogenic reefs
- Dynamics, threats and management of salt marshes
- Seagrass meadows
- Salt marshes
- Spatial and temporal variability of salt marshes
- Mangroves
- Shore protection vegetation
- Biogeomorphology of coastal systems
- Climate adaptation measures for the coastal zone
- Artificial reefs
- Ecological enhancement of coastal protection structures
- Blue carbon revenues of nature-based coastal protection
- Restoration of estuarine and coastal ecosystems
- Theseus Official Deliverable 2.1 - Integrated inventory of data and prototype experience on coastal defences and technologies
References
- ↑ Narayan, S., Beck, M., Reguero, B., Losada, I., Van Wesenbeeck, B., Pontee, N., Sanchirico, J., Ingram, J., Lange, G. and Burks-Copes, K. 2016. The Effectiveness, Costs and Coastal Protection Benefits of Natural and Nature-Based Defences. PLoS ONE 11, e0154735
- ↑ Van Coppenolle, R, and Temmerman, S. 2019. A global exploration of tidal wetland creation for nature-based flood risk mitigation in coastal cities. Estuarine, Coastal and Shelf Science 226, 106262
- ↑ Milchakova, N. A., Phillips, R. C., 2003. Black Sea seagrasses. Marine Pollution Bulletin. 46, 695-699.
- ↑ Duarte, C.M., 1991. Seagrass depth limits. Aquatic Botany.40(4), pp. 363-377.
- ↑ den Hartog, C., 1977. Structure, function and classification in sea grass communities. In: McRoy, C.P. and Hellferich, C. (eds.), 1977. Seagrass ecosystems. New York, Dekker. pp 89-121.
- ↑ 6.0 6.1 6.2 6.3 6.4 6.5 Koch, E.W., Sanford, L.P., Chen, S-N., Shafer, D.J., Mckee Smith, J., 2006. Waves in seagrass systems: Review and Technical recommendations. US Army Corps of Engineers®. Technical Report, ERDC TR-06-15.
- ↑ Borum, J., Duarte, C.M., Krause-Jensen, D., Greve, T.M., 2004. European seagrasses: an introduction to monitoring and management. Monitoring and Managing of European Seagrasses Project.
- ↑ Bradley, K. and Houser, C., 2009. Relative velocity of of seagrass blades: Implications for wave attenuation in low-energy environments. J. Geophys. Res. 114 F01004, doi:10.1029/2007JF000951.
- ↑ Mendez, F.J., Losada, I.J., 2004. An empirical model to estimate the propagation of random breaking and nonbreaking waves over vegetation fields. Coastal Engineering. 51(2), pp. 103-118.
- ↑ 10.0 10.1 Fonseca, M.S., Cahalan, J.H., 1992. A preliminary evaluation of wave attenuation by four species of Seagrass. Estuarine, Coastal and Shelf Science. 35(6), pp. 565-576.
- ↑ Bouma, T.J., De Vries, M.B., Low, E., Peralta, G., Tanczos, J.C., Van De Koppel, J., Herman, P.M.J., 2005. Trade-offs related to ecosystem engineering: A case study on stiffness of emerging macrophytes. Ecology. 86(8), 2187-2199.
- ↑ Fernando, H.J.S., Samarawickrama, S.P., Balasubramanian, S., Hettiarachchi, S.S.L., Voropayev, S., 2008. Effects of porous barriers such as coral reefs on coastal wave propagation. Journal of Hydro-environment Research. 1(3-4), pp. 187-194.
- ↑ Stratigaki V., Manca E., Prinos P., Losada I., Lara J., Sclavo M., Caceres I. , Sanchez-Arcilla A. (2010). “Large scale experiments on wave propagation over Posidonia oceanica, J. of Hydraulic Research IAHR [1]
- ↑ Paul, M. 2018. The protection of sandy shores – Can we afford to ignore the contribution of seagrass? Marine Pollution Bulletin 134: 152–159
- ↑ Borum, J., Duarte, C.M., Krause-Jensen, D., Greve, T.M., 2004. European seagrasses: an introduction to monitoring and management. Monitoring and Managing of European Seagrasses Project.
- ↑ Oprandi, A., Mucerino, L., De Leo, F., Bianchi, C.N., Morri, C., Azzola, A., Benelli, F., Besio, G., Ferrari, M. and Montefalcone, M., 2020. Effects of severe storm on seagrass meadows. Sci. Total Environ. 748, 141373
- ↑ Ward, L.G., Kemp, W.M., and Boynton, W.E., 1984. The influence of waves and seagrass communities on suspended particulates in an estuarine embayment. Marine Geology. 59, pp.85-103.
- ↑ Verduin, J. J., Backhaus, J. O., 2000. Dynamics of plant-flow interactions for the seagrass Amphibolis antarctica: Field observations and model simulations. Estuarine Coastal and Shelf Science. 50, p185-204.
- ↑ Granata, T.C., Serra, T., Colomer, J., Casamitjana, X., Duarte, C.M., Gacia, E., 2001. Flow and particle distributions in a nearshore seagrass meadow before and after a storm. Marine Ecology Progress Series. 218, pp. 95–106.
- ↑ Tigny, V., Ozer, A., De Falco, G., Baroli, M., and Djenidi, S., 2007. Relationship between the Evolution of the Shoreline and the Posidonia oceanica Meadow Limit in a Sardinian Coastal Zone. Journal of Coastal Research. 23, pp.797-793.
- ↑ Bradley, K., and Houser, C., 2009. Relative velocity of seagrass blades: Implications for wave attenuation in low-energy environments. J. Geophys. Res.. 114. F01004, doi:10.1029/2007JF000951.
- ↑ Ondiviela, B., Losada, I.J., Lara, J.L., Maza, M., Galván, C., Bouma, T.J. and van Belzen, J., 2014. The role of seagrasses in coastal protection in a changing climate. Coast. Eng. 87: 158–168
- ↑ 23.0 23.1 Short, F.T., Neckles, H.A., 1999. The effects of global climate change on seagrasses. Aquatic Botany. 63(3-4), Pages 169-196.
- ↑ Duarte, C.M., 1991. Seagrass depth limits. Aquatic Botany. 40(4), pp. 363-377.
- ↑ Borum, J., Duarte, C.M., Krause-Jensen, D., Greve, T.M., 2004. European seagrasses: an introduction to monitoring and management. Monitoring and Managing of European Seagrasses Project.
- ↑ Koch, E. and Beer, S., 1996. Tides, light and the distribution of the seagrass Zostera marina in Long Island Sound. Aquatic Botany 53, pp. 97–107.
- ↑ Björk, M., Short, F., Mcleod, E., Beer, S., 2008. Managing Seagrasses for Resilience to Climate Change. IUCN, Gland, Switzerland. 56pp.
- ↑ Adam P., 1990. Saltmarsh Ecology. Cambridge University Press, New York.
- ↑ Allen, J.R.L., 2000. Morphodynamics of Holocene salt marshes: a review sketch from the Atlantic and Southern North Sea coasts of Europe. Quaternary Science Reviews. 19(12), pp. 1155-1231.
- ↑ Janssen-Stelder, B.M., 2000. A synthesis of salt marsh development along the main land coast of the Dutch Wadden Sea. PhD thesis, University of Utrecht.
- ↑ Dijkema, K.S., 1987. Geography of salt marshes in Europe. Zeitschrift für Geomorphologie. 31(4), pp. 489-499.
- ↑ Chapman, V.J., 1976. Coastal vegetation. University of Auckland. Oxford Pergamon.
- ↑ 33.0 33.1 Allen, J.R.L., 2000. Morphodynamics of Holocene salt marshes: a review sketch from the Atlantic and Southern North Sea coasts of Europe. Quaternary Science Reviews. 19(12), pp. 1155-1231.
- ↑ 34.0 34.1 Pye, K., French, P.W., 1993. Erosion and accretion processes on British Salt Marshes. Vol. 1, Introduction: Saltmarsh Processes and Morphology. Cambridge Environmental Research Consultants, Cambridge.
- ↑ 35.0 35.1 Möller, I., 2006. Quantifying saltmarsh vegetation and its effect on wave height dissipation: Results from a UK East coast saltmarsh. Estuarine, Coastal and Shelf Science. 69(3-4), pp. 337-351.
- ↑ Möller, I., Spencer, T., French, J. R., Leggett, D. J., Dixon, M., 1999. Wave Transformation Over Salt Marshes: A Field and Numerical Modelling Study from North Norfolk, England. Estuarine, Coastal and Shelf Science. 49(3), pp. 411-426.
- ↑ Bouma, Herman, P.M.J., 2005. Trade-offs related to ecosystem engineering: A case study on stiffness of emerging macrophytes. Ecology. 86 (8), 2187-2199
- ↑ Figueroa-Alfaro, R.W., van Rooijen, A., Garzon, J.L., Evans, M. and Harris, A. 2022. Modelling wave attenuation by saltmarsh using satellite-derived vegetation properties. Ecological Engineering 176 (2022) 106528
- ↑ Zhang, M., Dai, Z., Bouma, T.J., Bricker, J., Townend, I., Wen, J., Zhao, T. and Cai, H. 2021. Tidal-flat reclamation aggravates potential risk from storm impacts. Coastal Engineering 166, 103868
- ↑ Vuik, V., Suh Heo, H.Y., Zhu, Z., Borsje, B.W. and Jonkman, S.N. 2018. Stem breakage of salt marsh vegetation under wave forcing: a field and model study. Estuar. Coast. Shelf Sci. 200: 41–58
- ↑ Grant, A. and Cooker, M.J. 2023. Reductions in water level over coastal wetlands during storm surges and tsunamis: An analytical result and a critical review of the literature. Coastal Engineering 183, 104328
- ↑ Vuik, V., Borsje, B.W., Willemsen, P.W.J.M. and Jonkman, S.N. 2019. Salt marshes for flood risk reduction: quantifying long-term effectiveness and life-cycle costs. Ocean Coast Manag. 171: 96–110
- ↑ Mi, J., Zhang, M., Zhu, Z., Vuik, V., Wen, J., Gao, H. and Bouma, T.J. 2022. Morphological wave attenuation of the nature-based flood defense: A case study from Chongming Dongtan Shoal, China. Science of the Total Environment 831, 154813
- ↑ Hughes, R.G. and Paramor, O.L.A., 2004. The effects of bioturbation and herbivory by the polychaete Nereis diversicolor on loss of saltmarsh in south-east England. Journal of Applied Ecology. 41, p440–448.
- ↑ Kiesel, J., Schuerch, M., Moeller, I., Spencer, C. and Vafeidis, A. 2019. Attenuation of high water levels over restored saltmarshes can be limited. Insights from Freiston Shore, Lincolnshire, UK. Ecological Engineering 136: 89–100
- ↑ Mazik, K., Musk,W., Dawes, O., Solyanko, K., Brown, Su., Mander, L. and Elliott, M., 2010. Managed realignment as compensation for the loss of intertidal mudflat: a short term solution to a long term problem? Estuar. Coast. Shelf Sci. 90: 11-20
- ↑ Mossman, H.L., Davy, A.J., Grant, A., 2012. Does managed coastal realignment create saltmarshes with ‘equivalent biological characteristics’ to natural reference sites? J. Appl. Ecol. 49; 1446-1456
- ↑ Pontee, N. 2014. Accounting for siltation in the design of intertidal creation schemes. Ocean and Coastal Management 88: 8-12
- ↑ Fagherazzi, S. 2013. The ephemeral life of a salt marsh. Geology 41: 943–944
- ↑ Kirwan, M.L., Walters, D.C., Reay, W.G. and Carr, J.A. 2016. Sea level driven marsh expansion in a coupled model of marsh erosion and migration. Geophys. Res. Lett. 43: 4366–4373
- ↑ Wood, R., 1999. Reef evolution. Oxford University Press, Oxford.
- ↑ Roberts, J.M., Wheeler, A.J., Freiwald, A., 2006. Reefs of the deep: The biology and geology of cold-water coral ecosystems. Science. 213, 543-547.
- ↑ Roberts, J.M., Davies, A.J., Henry, L.A., Duineveld, G., Lavaleye, M., Dodds, L.A., Maier, C., Van Soest, R.W.M., Bergman, M.I.N., Hühnerbach, V., Sinclair, D., Watmough, T., Long, D., Van Haren, H., 2009. The Mingulay reef complex, northeast Atlantic: an interdisciplinary study of cold-water coral habitat, hydrography and biodiversity. Marine Ecology Progress Series. 397, 139-151.
- ↑ 54.0 54.1 Holt, T.J., Rees, E.I., et al. 1998. Biogenic Reefs (volume IX). An overview of dynamic and sensitivity characteristics for conservation management of marine SACs. Scottish Association for Marine Science (UK Marine SACs Project). 170 Pages.
- ↑ Foster-Smith, R.L., Hendrick, V.J., 2003. Sabellaria spinulosa reef in The Wash and North Norfolk cSAC and its approaches: Part III, Summary of knowledge, recommended monitoring strategies and outstanding research requirements. English Nature Research Reports, 543.
- ↑ Hendrick, V.J., Foster-Smith, R.L., 2006. Sabellaria spinulosa reef: a scoring system for evaluating 'reefiness' in the context of the Habitats Directive. Journal of the Marine Biological Association of the United Kingdom. 86,665-677.
- ↑ 57.0 57.1 57.2 Wilson, D. P., 1971. Sabellaria colonies At Duckpool, North Cornwall, 1961-1970. Journal of the Marine Biological Association of the UK. 51, p509-580.
- ↑ 58.0 58.1 58.2 58.3 Gruet, Y., 1982. Recherches sur l‟écologie des récifs d'Hermelles édicés par l‟Annélide Polychète Sabellaria alveolata (Linné), Université des Sciences et Techniques, Nantes, France. PhD.
- ↑ 59.0 59.1 Cunningham, P.N., Hawkins, S.J., Jones, H.D., Burrows, M.T., 1984. The geographical distribution of Sabellaria alveolata (L.) in England, Wales and Scotland, with investigations into the community structure of, and the effects of trampling on Sabellaria alveolata colonies. Report to the Nature Conservancy Council from the Department of Zoology, Manchester University, Manchester.
- ↑ Mettam, C., Conneely, M.E., White, S.J., 1994. Benthic macrofauna and sediments in the Severn Estuary. Biological Journal of the Linnaean Society. 51, p71-81.
- ↑ Perkins, E.J., Abbott, O.J., Nottage, A.S., Reid, D.M., Lampard, D.J., 1980. Sixth annual report to the Cumbria Sea-Fisheries Committee. Solway Firth survey - 1st April 1979 to 31st March 1980. University of Strathclyde, Department of Biology.
- ↑ Marchand, Y., Cazoulat, R., 2003. Biological reef survey using spot satellite data classification by cellular automata method - Bay of Mont Saint-Michel (France). Computers & Geosciences. 29, p413-421.
- ↑ Poloczanska, E.S., Hughes, D.J., Burrows, M.T., 2004. Underwater television observations of Serpula vermicularis (L.) reefs and associated mobile fauna in Loch Creran, Scotland. Estuarine. Coastal and Shelf Science, 61, p425-435.
- ↑ Dodd, J., Baxter, L., Hughes, D., 2009. Mapping Serpula vermicularis (Polychaeta: Serpulidae) aggregations in Loch Teacuis, western Scotland, a new record. Mar Biol Res, 5, p200- 205.
- ↑ 65.0 65.1 Dankers, N., Brinkman, A.G., Meijboom, A., Dijkman, E., 2001. Recovery of intertidal mussel beds in the Waddensea: use of habitat maps in the management of the fishery. Hydrobiologia. 465, p21–30.
- ↑ Commito, J.A., Rusignuolo, B.R., 2000. Structural complexity in mussel beds: the fractal geometry of surface topography. Journal of Experimental Marine Biology and Ecology. 255, p133–152.
- ↑ Commito, J.A., Dankers, N., 2001. Dynamics of spatial and temporal complexity in European and North American softbottom mussel beds. In: Reise, K. (Editor). Ecological Comparisons of Sedimentary Shores. Springer-Verlag, Heidelberg, pp. 39–59.
- ↑ 68.0 68.1 68.2 Widdows, J., Brinsley, M.D., 2002. Impact of biotic and abiotic processes on sediment dynamics and the consequences to the structure and functioning of the intertidal zone. Journal of Sea Research. 48, p143–156.
- ↑ 69.0 69.1 Widdows, J., Donkin, P., Staff, F.J., Matthiessen, P., Law, R.J., Allen, Y.T., Thain, J.E., Allchin, C.R., Jones, B.R., 2002. Measurement of stress effects (scope for growth) and contaminant levels in mussels (Mytilus spp.) collected from the Irish Sea. Marine Environmental Research. 53, p327–356.
- ↑ Gutiérrez, J.L., Jones, C.G., Strayer, D.L., Iribarne, O.O., 2003. Mollusks as ecosystem engineers: the role of shell production in aquatic habitats. Oikos. 101, p79–90.
- ↑ 71.0 71.1 Flemming, B.W., Delafontaine, M.T., 1994. Biodeposition in a juvenile mussel bed of the East Frisian Wadden Sea (southern North Sea). Netherlands Journal of Aquatic Ecology, 28, p289–297.
- ↑ Oost, A.P., 1995. The influence of biodeposits of the blue mussel Mytilus spp. on fine- grained sedimentation in the temperate-climate Dutch Wadden Sea. Geologica Ultraiectina. 126, p359-400.
- ↑ Costanza, R., d’Arge, R., de Groot, R., Farber, S., Grasso, M., Hannon, B., Limburg, K., Naeem, S., O’Neill, R.V., Paruelo, J., Raskin, R.G., Sutton, P., van den Belt, M., 1997. The value of the world’s ecosystem services and natural capital, Nature. 387, pp. 253-260.
- ↑ De Groot, R. Stuip, M., Finlayson, M. and N. Davidson, 2002. Valuing wetlands: Guidance for valuing the benefitsderived from wetland ecosystem services. Ramsar Technical Report No. 3, CBD Technical Series No. 27. [2]
- ↑ Moberg, F., Rönnbäck, P., 2003. Ecosystem services of the tropical seascape: interactions, substitutions and restoration. Ocean & Coastal Management. 46, p27-46
- ↑ Bianchi, C.N., 2001. La biocostruzione negli ecosistemi marini e la biologia marina Italiana. Biologia Marina Mediterranea. 8, p112-130.
- ↑ Dubois, S., et al., 2002. Biodiversity associated with Sabellaria alveolata (Polychaeta: Sabellariidae) reefs: effects of human disturbances. Journal of the Marine Biological Association of the UK. 82(05), p817-826.
- ↑ O'Connor, N.E., Crowe, T.P., 2007. Biodiversity among mussels: separating the influence of sizes of mussels from the ages of patches. Journal of the Marine Biological Association of the United Kingdom. 89, p551-557.
- ↑ 79.0 79.1 79.2 Jackson, A., Hiscock, K., 2008. Sabellaria spinulosa. Ross worm. Marine Life Information Network: Biology and Sensitivity Key Information Sub-programme [on-line]. Plymouth: Marine Biological Association of the United Kingdom. [cited 28/04/2010]. Available from: [3]
- ↑ Hendrick, V.J., Foster-Smith, R.L., 2006 Sabellaria spinulosa reef: a scoring system for evaluating 'reefiness' in the context of the Habitats Directive. Journal of the Marine Biological Association of the United Kingdom. 86, p665-677.
- ↑ Davies, A.J., Last, K.S., Attard, K., Hendrick, V.J., 2009. Maintaining turbidity and current flow in laboratory aquarium studies, a case study using Sabellaria spinulosa. Journal of Experimental Marine Biology and Ecology. 370, p35-40.
- ↑ Fager, E.W., 1964. Marine Sediments: Effects of a Tube-Building Polychaete. Science. 143(3604), 356-358.
- ↑ Gram, R., 1968. A Florida Sabellaridae reef and its effect on sediment distribution. J. Sediment. Petrol., 38: 863- 868.
- ↑ Multer, H.G., Milliman, J.G., 1967. Geologic aspects of sabellarian reefs, Southeastern Florida. Bulletin of Marine Science. 17. p257- 267.
- ↑ Naylor, L.A., Viles, H.A., 2000. A temperate reef builder: an evaluation of the growth, morphology and composition of Sabellaria alveolata (L.) colonies on carbonate platforms in South Wales. In: E.R. Insalaco, P.W. Skelton and T.J. Palmer (Eds), 2000. Carbonate platform systems: components and interactions. Spec. Publ.-Geol. Soc. Lond. vol. 178 (2000), pp. 9–19.
- ↑ Naylor, L.A., 2001. An assessment of the links between biogenic processes and shore platform geomorphology. Glamorgan Heritage Coast, South Wales, UK. Thesis, D. Phil., University of Oxford.
- ↑ Crisp, D.J., 1964. The effects of the severe winter of 1962-63 on marine life in Britain. Journal of Animal Ecology. 33, p165-210.
- ↑ Beukema, J.J., Cadée, G.C., 1996. Consequences of the sudden removal of nearly all mussels and cockles from the Dutch Wadden Sea. PSZN Mar Ecol. 17, p279–289.
- ↑ van Katwijk, M.M., 2003. Reintroduction of eelgrass (Zostera marina L.) in the Dutch Wadden Sea, a research overview and management vision. p.173-197. In: Challenges to the Wadden Sea area. Wolff W.J., K. Essink, A. Kellermann and M.A. van Leeuwe (Eds). Proceedings of the 10th International Scientific Wadden Sea Symposium.
- ↑ De Vries, M.B., Bouma, T.J., van Katwijk, M.M., Borsje, B.W., Van Wesenbeeck, B.K., 2007. Biobouwers van de kust. Report Z4158. WL|Delft Hydraulics, Delft, The Netherlands.
- ↑ van Leeuwen, B., Augustijn, D.C.M., et al., 2010. Modeling the influence of a young mussel bed on fine sediment dynamics on an intertidal flat in the Wadden Sea. Ecological Engineering. 36(2), p145-153.
- ↑ Seed,, R., Suchanek T.H., 1992. Population and community ecology of Mytilus. Amsterdam, Elsevier Science Publ.
- ↑ Odum, H.T., Odum, B., 2003. Concepts and methods of ecological engineering. Ecological Engineering. 20, p339–361.
Please note that others may also have edited the contents of this article.
|
Please note that others may also have edited the contents of this article.
|









