Difference between revisions of "Mud"
Dronkers J (talk | contribs) |
|||
| (41 intermediate revisions by 6 users not shown) | |||
| Line 1: | Line 1: | ||
| − | == | + | {{Review |
| − | + | |name=Jean Berlamont | |
| − | + | |AuthorID=49 | |
| − | + | }} | |
| − | + | ||
| − | + | {{ | |
| − | + | Definition|title=Mud | |
| − | + | |definition= Fine cohesive sediment deposit containing a high fraction (≥20%) of clay minerals which cause the sediment to bind together. }} | |
| + | |||
| + | See also: [[Definitions of coastal terms#Mud]] | ||
| + | |||
| + | |||
| + | Fine materials, such as [[clay]] and [[silt]], also referred to as mud, do not normally constitute a stable coastal profile if exposed to even moderate wave action. Consequently, such materials are normally kept in suspension by the waves until they settle in deep water. [[Flocculation cohesive sediments|Flocculation]] influences the siltation process in the mixing zone between freshwater and sea-water, where stratification also occurs. | ||
| + | |||
| − | [[Image:mud.jpg| | + | ==Some basic properties== |
| + | [[Image:mud.jpg|thumb|mud!]] | ||
| + | Particle Size: | ||
| + | *''[[Silt]]'': Grain size .002-.063mm. | ||
| + | *''[[Clay]]'': Grain size is typically less than .002mm. Possesses electromagnetic properties that bind grains together to give bulk strength or cohesion. | ||
| + | Ionic charges: | ||
| + | *Interact electrostatically. | ||
| + | Additionally: | ||
| + | *Organic material influences sediment properties | ||
| + | *There is a strong sediment-fluid interaction in mud ([[Flocculation cohesive sediments|flocculation]], turbulence damping etc.) | ||
| − | + | Below is a figurative description of the cohesive sediment mechanism. | |
| + | [[Image:cohesive sediment.jpg|center|450px|cohesive sediment mechanism]] | ||
==The nature of flocculation== | ==The nature of flocculation== | ||
| + | ''The following text is taken from Karen Edelvang (1995) "The significance of aggregation in an estuarine environment." PhD thesis printed in Geographica Hafniensia A5 ISBN 87-87945-18-5'' | ||
| − | Flocculation is governed by a number of processes that all influence the nature of the single particles forming part of the floc or aggregate. | + | [[Flocculation cohesive sediments|Flocculation]] is governed by a number of processes that all influence the nature of the single particles forming part of the floc or aggregate. |
===Mineralogy=== | ===Mineralogy=== | ||
| − | <ref name="KAE">Edelvang, K. 1995. "The significance of aggregation in an estuarine | + | <ref name="KAE">Edelvang, K. 1995. "The significance of aggregation in an estuarine environment." PhD thesis printed in Geographica Hafniensia A5 ISBN 87-87945-18-5</ref>Flocs are an assemblage of minerals normally representing varying minerals and grain sizes. Particles of [[clay]] minerals are the major constituents in flocs. The mineralogic composition of these particles plays a role in the process of flocculation. The most resistant material in the aquatic environment is quartz, which is found in all particle fractions down to less than 2 μm. Quartz has no cohesive properties and does not take part in the flocculation processes directly, but might well be part of aggregates originating from fecal pellets. Especially in spring, algal blooms (see: [[Eutrophication in coastal environments|Eutrophication]]) lead to a large input of diatoms thus giving high concentrations of silicate in the suspended matter. |
| − | |||
| − | |||
| − | |||
| + | The most abundant inorganic material found in suspension is weathered products of mica and feldspars. The most common [[clay]] minerals are kaolinite, illite and montmorillonite. Quartz particles may also be suspended under rough turbulent conditions. Kaolinite is a dimorphic (1:1 layers) mineral constituting of a silica (tetrahedral) sheet and an alumina (octahedral) sheet. Illite and montmorillonite are trimorphic (2:1 layers) with two silica sheets sandwiching an alumina sheet. | ||
===Chemical properties of clay particles=== | ===Chemical properties of clay particles=== | ||
| − | <ref name="KAE"/>Clay particles have plate-like features and an overall negative ionic charge due to broken mineral bonds on their faces. This means that in a solution the negatively charged clay particles are attracted by positive electrodes. The internal balance in a solution, which has no net electric charge, is internally compensated for by the electric double layer. The double layer consists of the negative clay particle charge and the equivalent amount of cationic charge attracted by the clay particle and accumulated in the liquid near the particle surface<ref> Van Olphen H (1963) An introduction to clay colloid chemistry. Interscience Publishers (John Wiley & Sons) pp 301 | + | <ref name="KAE"/>[[Clay]] particles have plate-like features and an overall negative ionic charge due to broken mineral bonds on their faces. This means that in a solution the negatively charged [[clay]] particles are attracted by positive electrodes. The internal balance in a solution, which has no net electric charge, is internally compensated for by the electric double layer. The double layer consists of the negative clay particle charge and the equivalent amount of cationic charge attracted by the [[clay]] particle and accumulated in the liquid near the particle surface<ref> Van Olphen H (1963) An introduction to [[clay]] colloid chemistry. Interscience Publishers (John Wiley & Sons) pp 301 |
| − | </ref>. The accumulated cations around the clay particle are called counter-ions. | + | </ref>. The accumulated cations around the [[clay]] particle are called counter-ions. |
====Brownian Motion==== | ====Brownian Motion==== | ||
| − | <ref name="KAE"/>The Brownian motion is the irregular motion of any particle in suspension. The Brownian motion is caused by the thermal motion of water molecules colliding with the particle thus changing its direction randomly. Increased particle size means that the Brownian motion become less vivid as more kinetic energy is required to set the particle in motion or change its path | + | <ref name="KAE"/>The Brownian motion is the ''irregular motion of any particle in suspension''. The Brownian motion is caused by the thermal motion of water molecules colliding with the particle thus changing its direction randomly. Increased particle size means that the Brownian motion become less vivid as more kinetic energy is required to set the particle in motion or change its path. |
| − | |||
| − | |||
| − | |||
| + | The Brownian motion leads to the suspended particles randomly approaching one another until their counter-ions interfere. Consequently the particles repel each other. The amount of work involved in this repulsion is the repulsive potential, Vr, also called the Coulombic force<ref>Dyer KR (1986) Coastal and Estuarine Sediment Dynamics. John Wiley & Sons 342 pp</ref> decreasing exponentially with the distance between the particles. Particles in saline water tend to flocculate and form large aggregates in spite of the repulsive forces still present. | ||
===Size distribution of single particles=== | ===Size distribution of single particles=== | ||
| − | <ref name="KAE"/>Size distribution of suspended matter varies according to the environment. Clay particles act as colloids, defined as particles mainly less than 2 μm with large surface areas per unit mass<ref> Edzwald JK & O'Melia CR (1975) Clay Distribution in Recent Estuarine Sediments. Clay and Clay minerals, Vol 23 p 39-44 | + | <ref name="KAE"/>Size distribution of suspended matter varies according to the environment. [[Clay]] particles act as colloids, defined as particles mainly less than 2 μm with large surface areas per unit mass<ref> Edzwald JK & O'Melia CR (1975) Clay Distribution in Recent Estuarine Sediments. Clay and Clay minerals, Vol 23 p 39-44</ref>. They are chemically active because of their broken mineral bonds causing isomorph substitution in the crystal lattice. Normally the size range of the single particles forming the flocs is between less than 2 μm up to 20-30 μm thus in the clay- and silt-fraction. The different minerals seem to have limited and differing size ranges in the natural environment as shown by Gibbs<ref name="Gibbs"> Gibbs RJ (1977) Clay Mineral Segregation in the Marine Environment. Journal of Sed Pet, Vol 47, p 237-243</ref>. This is of importance when looking at the sorting, which naturally takes place in the estuarine environment<ref name="Gibbs"/>. |
| − | </ref>. They are chemically active because of their broken mineral bonds causing isomorph substitution in the crystal lattice. Normally the size range of the single particles forming the flocs is between less than 2 μm up to 20-30 μm thus in the clay- and silt- | ||
| − | |||
| − | The different minerals seem to have limited and differing size ranges in the natural | ||
===The biological component: fecal pellets=== | ===The biological component: fecal pellets=== | ||
| Line 51: | Line 61: | ||
===Aggregate properties=== | ===Aggregate properties=== | ||
| − | <ref name="KAE"/>Flocculation is governed by increasing concentration in a suspension. Aggregates tend to be more fragile the larger they become having lower densities, yield strength and viscosity allowing them to break up more easily. Flocs are stated to be self-similar in structure regardless of size. Thus the basic structure of the individual element is thought to be geometrically repeated in a hierarchial process. The fractal dimension is described by a value, D: | + | <ref name="KAE"/>Flocculation is governed by increasing concentration in a suspension. Aggregates tend to be more fragile the larger they become having lower densities, yield strength and viscosity allowing them to break up more easily. Flocs are stated to be self-similar in structure regardless of size. Thus the basic structure of the individual element is thought to be geometrically repeated in a hierarchial process. The fractal dimension is described by a value, <math>D</math>: |
| + | |||
| − | D = ln | + | <math>D = \ln(m_1)/ln(m_2)</math>, |
| − | where | + | where <math>m_1</math> = the number of primary particles in a fixed structure, <math>m_2</math>= the factor by which the size of the aggregate increases. <math>D</math> is found to vary between 1 for polarized particles, 1.8 for flocs formed by collision to 2.1 for particles flocculating in suspensions with no electrolyte dissolved. Flocs with <math>D > 2</math> are compact in structure, flocs with <math>D < 2</math> are more fragile. |
| + | |||
| + | Yield strength is also an important parameter in the description of mud properties. | ||
| + | |||
| + | ===Settling velocities=== | ||
| + | <ref name="KAE"/>Migniot (1968)<ref> Migniot C (1968) Etude des propriétés physiques de differents sediments trés fins et de leur comportement sous des actions hydrodynamiques. Houille Blanche 7 p 591-620</ref>mentions flocculation and hence floc settling velocity as one of the three basic physical properties of suspensions. Flocs and fecal pellets have physical properties which separate them from single particles. It is often argued that settling velocity rather than shape or equivalent fall diameter should be used to describe aggregates. However, it is difficult to measure settling velocity in situ. | ||
| − | |||
| − | |||
| − | |||
| − | + | ==Related articles== | |
| + | :[[Dynamics of mud transport]] | ||
| + | :[[Sediment deposition and erosion processes]] | ||
| + | :[[Characteristics of muddy coasts]] | ||
| + | :[[Coastal and marine sediments]] | ||
| + | :[[Flocculation cohesive sediments]] | ||
| + | :[[Fluid mud]] | ||
| + | :[[Coastal mud belt]] | ||
| − | |||
| − | |||
| − | |||
==References== | ==References== | ||
<references/> | <references/> | ||
| − | |||
| − | == | + | {{author |
| + | |AuthorID=11539 | ||
| + | |AuthorFullName=Edelvang, Karen | ||
| + | |AuthorName=Karen Edelvang}} | ||
| − | + | [[Category:Physical coastal and marine processes]] | |
| + | [[Category:Sediment]] | ||
Latest revision as of 16:12, 14 February 2024
Definition of Mud:
Fine cohesive sediment deposit containing a high fraction (≥20%) of clay minerals which cause the sediment to bind together.
This is the common definition for Mud, other definitions can be discussed in the article
|
See also: Definitions of coastal terms#Mud
Fine materials, such as clay and silt, also referred to as mud, do not normally constitute a stable coastal profile if exposed to even moderate wave action. Consequently, such materials are normally kept in suspension by the waves until they settle in deep water. Flocculation influences the siltation process in the mixing zone between freshwater and sea-water, where stratification also occurs.
Contents
Some basic properties
Particle Size:
- Silt: Grain size .002-.063mm.
- Clay: Grain size is typically less than .002mm. Possesses electromagnetic properties that bind grains together to give bulk strength or cohesion.
Ionic charges:
- Interact electrostatically.
Additionally:
- Organic material influences sediment properties
- There is a strong sediment-fluid interaction in mud (flocculation, turbulence damping etc.)
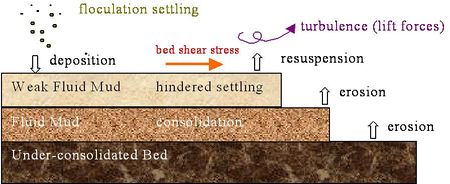
Below is a figurative description of the cohesive sediment mechanism.
The nature of flocculation
The following text is taken from Karen Edelvang (1995) "The significance of aggregation in an estuarine environment." PhD thesis printed in Geographica Hafniensia A5 ISBN 87-87945-18-5
Flocculation is governed by a number of processes that all influence the nature of the single particles forming part of the floc or aggregate.
Mineralogy
[1]Flocs are an assemblage of minerals normally representing varying minerals and grain sizes. Particles of clay minerals are the major constituents in flocs. The mineralogic composition of these particles plays a role in the process of flocculation. The most resistant material in the aquatic environment is quartz, which is found in all particle fractions down to less than 2 μm. Quartz has no cohesive properties and does not take part in the flocculation processes directly, but might well be part of aggregates originating from fecal pellets. Especially in spring, algal blooms (see: Eutrophication) lead to a large input of diatoms thus giving high concentrations of silicate in the suspended matter.
The most abundant inorganic material found in suspension is weathered products of mica and feldspars. The most common clay minerals are kaolinite, illite and montmorillonite. Quartz particles may also be suspended under rough turbulent conditions. Kaolinite is a dimorphic (1:1 layers) mineral constituting of a silica (tetrahedral) sheet and an alumina (octahedral) sheet. Illite and montmorillonite are trimorphic (2:1 layers) with two silica sheets sandwiching an alumina sheet.
Chemical properties of clay particles
[1]Clay particles have plate-like features and an overall negative ionic charge due to broken mineral bonds on their faces. This means that in a solution the negatively charged clay particles are attracted by positive electrodes. The internal balance in a solution, which has no net electric charge, is internally compensated for by the electric double layer. The double layer consists of the negative clay particle charge and the equivalent amount of cationic charge attracted by the clay particle and accumulated in the liquid near the particle surface[2]. The accumulated cations around the clay particle are called counter-ions.
Brownian Motion
[1]The Brownian motion is the irregular motion of any particle in suspension. The Brownian motion is caused by the thermal motion of water molecules colliding with the particle thus changing its direction randomly. Increased particle size means that the Brownian motion become less vivid as more kinetic energy is required to set the particle in motion or change its path.
The Brownian motion leads to the suspended particles randomly approaching one another until their counter-ions interfere. Consequently the particles repel each other. The amount of work involved in this repulsion is the repulsive potential, Vr, also called the Coulombic force[3] decreasing exponentially with the distance between the particles. Particles in saline water tend to flocculate and form large aggregates in spite of the repulsive forces still present.
Size distribution of single particles
[1]Size distribution of suspended matter varies according to the environment. Clay particles act as colloids, defined as particles mainly less than 2 μm with large surface areas per unit mass[4]. They are chemically active because of their broken mineral bonds causing isomorph substitution in the crystal lattice. Normally the size range of the single particles forming the flocs is between less than 2 μm up to 20-30 μm thus in the clay- and silt-fraction. The different minerals seem to have limited and differing size ranges in the natural environment as shown by Gibbs[5]. This is of importance when looking at the sorting, which naturally takes place in the estuarine environment[5].
The biological component: fecal pellets
[1]Benthic organisms tend to modify cohesive sediment characteristics. Individual particles are ingested and form large, low density fecal pellets with settling velocities much higher than the individual particles contained in them. Estuarine sediment transport conditions are altered e.g. by change of bed roughness due to deposition of fecal pellets or by biological activity in the surface layers causing either binding or destabilizing of the bed surface[6].
Density, size and settling velocity of fecal pellets are important in describing sediment transport processes[7]. Fecal pellets are low density feces from suspension-feeding and mud-ingesting marine and estuarine benthic animals including oysters, worms, and barnacles. They consist of large amounts of fine sediments mainly single particles in the 1-5 μm range[8] and flocs ingested by the feeding animal, compacted in the gut and excreted as large aggregates glued together by mucus. Up to 25 % of the deposited sediment may in some areas originate from biodeposition.
Aggregate properties
[1]Flocculation is governed by increasing concentration in a suspension. Aggregates tend to be more fragile the larger they become having lower densities, yield strength and viscosity allowing them to break up more easily. Flocs are stated to be self-similar in structure regardless of size. Thus the basic structure of the individual element is thought to be geometrically repeated in a hierarchial process. The fractal dimension is described by a value, [math]D[/math]:
[math]D = \ln(m_1)/ln(m_2)[/math],
where [math]m_1[/math] = the number of primary particles in a fixed structure, [math]m_2[/math]= the factor by which the size of the aggregate increases. [math]D[/math] is found to vary between 1 for polarized particles, 1.8 for flocs formed by collision to 2.1 for particles flocculating in suspensions with no electrolyte dissolved. Flocs with [math]D \gt 2[/math] are compact in structure, flocs with [math]D \lt 2[/math] are more fragile.
Yield strength is also an important parameter in the description of mud properties.
Settling velocities
[1]Migniot (1968)[9]mentions flocculation and hence floc settling velocity as one of the three basic physical properties of suspensions. Flocs and fecal pellets have physical properties which separate them from single particles. It is often argued that settling velocity rather than shape or equivalent fall diameter should be used to describe aggregates. However, it is difficult to measure settling velocity in situ.
Related articles
- Dynamics of mud transport
- Sediment deposition and erosion processes
- Characteristics of muddy coasts
- Coastal and marine sediments
- Flocculation cohesive sediments
- Fluid mud
- Coastal mud belt
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 Edelvang, K. 1995. "The significance of aggregation in an estuarine environment." PhD thesis printed in Geographica Hafniensia A5 ISBN 87-87945-18-5
- ↑ Van Olphen H (1963) An introduction to clay colloid chemistry. Interscience Publishers (John Wiley & Sons) pp 301
- ↑ Dyer KR (1986) Coastal and Estuarine Sediment Dynamics. John Wiley & Sons 342 pp
- ↑ Edzwald JK & O'Melia CR (1975) Clay Distribution in Recent Estuarine Sediments. Clay and Clay minerals, Vol 23 p 39-44
- ↑ 5.0 5.1 Gibbs RJ (1977) Clay Mineral Segregation in the Marine Environment. Journal of Sed Pet, Vol 47, p 237-243
- ↑ Nowell ARM, Jumars PA & Eckman JE (1981) Effects of biological activity on the entrainment of marine sediments. Mar Geol 42, p 133-153
- ↑ Taghon GL, Nowell ARM & Jumars PA (1984) Transport and breakdown of fecal pellets: Biological and sedimentological consequences. Limnol Oceanogr Vol 29(1), p 64-72
- ↑ Haven DS & Morales-Alamo R (1972) Biodeposition as a factor in sedimentation of fine suspended solids in estuaries. Geol Soc Mem, Vol 133, p 121-130
- ↑ Migniot C (1968) Etude des propriétés physiques de differents sediments trés fins et de leur comportement sous des actions hydrodynamiques. Houille Blanche 7 p 591-620
Please note that others may also have edited the contents of this article.
|