Difference between revisions of "Chemical ecology"
(→See also) |
Dronkers J (talk | contribs) |
||
| (14 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
==The importance of chemical ecology== | ==The importance of chemical ecology== | ||
| − | Chemical ecology | + | Chemical ecology has helped to understand terrestrial ecosystems. How bees pollinate flowers, how birds find their nests and human attractiveness to a partner are some of the many examples of interactions which are mediated by chemicals. It is not difficult to imagine the catastrophic consequences of the absence of such crucial relationships. |
Imagine a similar scenario without chemical interactions in the marine environment. | Imagine a similar scenario without chemical interactions in the marine environment. | ||
| − | Species would no longer be able to identify their food, locate their pray, recognize mates,... . | + | [[Species]] would no longer be able to identify their food, locate their pray, recognize mates,... . Species-specific chemicals can shape community processes such as seasonal succession, [[niche]] structure, selective feeding and [[population]] dynamics. |
| − | The [http://www.marBEF.org MarBEF] | + | The [http://www.marBEF.org MarBEF] ROSEMEB (Role of Secondary Metabolites in Ecosystem Biodiversity) project has provided a better understanding of the roles of these chemicals in maintaining [[Marine_Biodiversity|marine biodiversity]] and driving [[ecosystem function|ecosystem functionality]]. Some of these findings are discussed bellow<ref name="ma">[https://www.researchgate.net/publication/306030378_Marine_Biodiversity_and_Ecosystem_Functioning Heip, C., Hummel, H., van Avesaath, P., Appeltans, W., Arvanitidis, C., Aspden, R., Austen, M., Boero, F., Bouma, TJ., Boxshall, G., Buchholz, F., Crowe, T., Delaney, A., Deprez, T., Emblow, C., Feral, JP., Gasol, JM., Gooday, A., Harder, J., Ianora, A., Kraberg, A., Mackenzie, B., Ojaveer, H., Paterson, D., Rumohr, H., Schiedek, D., Sokolowski, A., Somerfield, P., Sousa Pinto, I., Vincx, M., Węsławski, JM., Nash, R. (2009). Marine Biodiversity and Ecosystem Functioning. Printbase, Dublin, Ireland ISSN 2009-2539]</ref>. |
<P> | <P> | ||
<BR> | <BR> | ||
| Line 11: | Line 11: | ||
===Chemical ecology and microbes=== | ===Chemical ecology and microbes=== | ||
| − | Microbes sense their environment via cell-associated and diffusible molecules such as AHL | + | Microbes sense their environment via cell-associated and diffusible molecules such as AHL (N-acylhomoserine lactones). Such molecules are constantly produced by many bacteria and diffuse through membranes into the surrounding environment. |
| − | (N-acylhomoserine lactones). Such molecules are constantly produced by many bacteria and diffuse through membranes into the surrounding environment. | ||
| − | When a certain cell density (a threshold or quorum) of the bacterial population and a corresponding concentration of AHL is reached, the expression of certain target genes is initiated. | + | When a certain cell density (a threshold or quorum) of the bacterial population and a corresponding concentration of AHL is reached, the expression of certain target genes is initiated. The proteins expressed by these genes may include the proteins for light emission in luminous bacteria or pathogenic factors that cause disease. |
| − | This quorum-sensing typically controls processes, such as swarming (coordinated movement), virulence (coordinated attack) or conjugation (gene transfer between cells), which require high cell densities for success and that are essential for the survival of the organisms which produce the molecules | + | This quorum-sensing typically controls processes, such as swarming (coordinated movement), virulence (coordinated attack) or conjugation (gene transfer between cells), which require high cell densities for success and that are essential for the survival of the organisms which produce the molecules<ref name="ma"/>. |
<P> | <P> | ||
<BR> | <BR> | ||
<P> | <P> | ||
| + | |||
===Chemical ecology and phytoplankton=== | ===Chemical ecology and phytoplankton=== | ||
| − | Many [[plankton]] species use a chemical defence against their predators, either through toxin production or feeding deterrence. [http://www.marinespecies.org/aphia.php?p=taxdetails&id=148898 Diatoms] are key players at the base of the marine [[food web]] and have always been assumed to be a good food source for herbivores. Some species however use chemicals as a defence against being grazed. | + | Many [[Marine_Plankton|plankton]] species use a chemical defence against their predators, either through toxin production or feeding deterrence. [http://www.marinespecies.org/aphia.php?p=taxdetails&id=148898 Diatoms] are key players at the base of the marine [[food web]] and have always been assumed to be a good food source for herbivores. Some species however use chemicals as a defence against being grazed. |
The discovery that these unicellular [[algae]] produced chemicals, such as polyunsaturated aldehydes (PUAs) and other oxidised products of fatty acid metabolism (collectively termed oxylipins), that induced abortions, birth defects, poor development and high offspring mortality to their grazers has changed our view of plant-animal interactions in the plankton. (C on the figure) | The discovery that these unicellular [[algae]] produced chemicals, such as polyunsaturated aldehydes (PUAs) and other oxidised products of fatty acid metabolism (collectively termed oxylipins), that induced abortions, birth defects, poor development and high offspring mortality to their grazers has changed our view of plant-animal interactions in the plankton. (C on the figure) | ||
[[Image:13.JPG|thumb|centre|700px| <div> | [[Image:13.JPG|thumb|centre|700px| <div> | ||
| − | The sponge [http://www.marinespecies.org/aphia.php?p=taxdetails&id=133911 ''Aplysina aerophoba''] produces the anti-microbial and anti-tumour compound Aplysinin-1. Staining with DAPI reveals the rich microbial fauna associated with the sponge. B. The effect of diatom-derived unsaturated aldehydes on diatoms (a) [http://www.marinespecies.org/aphia.php?p=taxdetails&id=175584 Phaeodactylum tricornutum] and (b) [http://www.marinespecies.org/aphia.php?p=taxdetails&id=163513 Thalassiosira weissflogii]. C. The effect of the diatom-derived unsaturated aldehydes 2-trans-4- cis-decatrienal (A1), 2-trans-4-trans-7-cis-decatrienal (A2) and 2-trans-4trans-decadienal (A3) on copepod hatching success | + | The sponge [http://www.marinespecies.org/aphia.php?p=taxdetails&id=133911 ''Aplysina aerophoba''] produces the anti-microbial and anti-tumour compound Aplysinin-1. Staining with DAPI reveals the rich microbial fauna associated with the sponge. B. The effect of diatom-derived unsaturated aldehydes on diatoms (a) [http://www.marinespecies.org/aphia.php?p=taxdetails&id=175584 ''Phaeodactylum tricornutum''] and (b) [http://www.marinespecies.org/aphia.php?p=taxdetails&id=163513 ''Thalassiosira weissflogii'']. C. The effect of the diatom-derived unsaturated aldehydes 2-trans-4- cis-decatrienal (A1), 2-trans-4-trans-7-cis-decatrienal (A2) and 2-trans-4trans-decadienal (A3) on copepod hatching success. D,E. Transformation of caulerpenyne in the allomonal oxytoxins by lipolytic enzymes, named Lip-1 (Lipase 1) and Lip-2 (Lipase 2), in [http://www.marinespecies.org/aphia.php?p=taxdetails&id=140672 ''Oxynoe olivacea''].</div>]] |
| − | Although | + | Although these toxins don't cause death, they can have ecological effects. They can sabotage future generations of grazers and therefore allow [[Algal_bloom|diatom blooms]] to persist when grazing pressure would normally have caused them to crash. This defence mechanism is new and specific for the marine environment. In contrast, most of the known (terrestrial) negative plant–animal interactions are related to poisoning, repellence or feeding deterrence instead of to reproductive failure. |
| − | In fact, the production of PUAs mainly affects future generations of grazers and have less | + | In fact, the production of PUAs mainly affects future generations of grazers and have less effects on the direct adult grazers. PUAs have also been shown to negatively impact other [[phytoplankton]] cells where they might function as a signal to trigger active cell-death. (B on the figure) |
So, these compounds may have multiple functions within plankton communities. They can act as defence molecules against predators and competitors, as well as signal molecules to drive diatom bloom dynamics and species succession patterns. More information can be found [[Functional_metabolites_in_phytoplankton#Phytoplankton-zooplankton_chemical_interactions|here.]] | So, these compounds may have multiple functions within plankton communities. They can act as defence molecules against predators and competitors, as well as signal molecules to drive diatom bloom dynamics and species succession patterns. More information can be found [[Functional_metabolites_in_phytoplankton#Phytoplankton-zooplankton_chemical_interactions|here.]] | ||
| − | Other phytoplankton groups such as the [http://www.marinespecies.org/aphia.php?p=taxdetails&id=146202 dinoflagellates] produce neurotoxins that can be transferred ([[biomagnification|biomagnify]]) up the marine [[food chain]] and have been responsible for mass fish-kills | + | Other phytoplankton groups such as the [http://www.marinespecies.org/aphia.php?p=taxdetails&id=146202 dinoflagellates] produce neurotoxins that can be transferred ([[biomagnification|biomagnify]]) up the marine [[food chain]] and have been responsible for mass fish-kills, as well as for the deaths of [[pollution and sea birds| sea birds]] and [[pollution and marine mammals|marine mammals]], including whales and sea lions. See also [[Harmful_algal_blooms|here]]. |
In humans, consumption of shellfish containing high levels of such toxins can induce paralytic, neurotoxic, diarrhetic and amnesic shellfish poisoning. Records of human poisoning by at least two of these syndromes date back hundreds of years, yet the discovery and characterisation of the responsible molecules happened quite recent. | In humans, consumption of shellfish containing high levels of such toxins can induce paralytic, neurotoxic, diarrhetic and amnesic shellfish poisoning. Records of human poisoning by at least two of these syndromes date back hundreds of years, yet the discovery and characterisation of the responsible molecules happened quite recent. | ||
| − | Many [[benthic]] invertebrates can use compounds | + | Many [[benthic]] invertebrates can use compounds from the food they consume, as defensive molecules against predators. Some plankton species might possibly do the same. |
| − | Lots of research still needs to be conducted on the effects of toxins on gamete, embryonic and larval development of herbivorous grazers | + | Lots of research still needs to be conducted on the effects of toxins on gamete, embryonic and larval development of herbivorous grazers<ref name="ma"/>. |
<P> | <P> | ||
<BR> | <BR> | ||
<P> | <P> | ||
| + | |||
===Chemical ecology and seaweeds=== | ===Chemical ecology and seaweeds=== | ||
| − | Seaweeds have been shown to produce a large variety of metabolites with highly variable structures (such as terpenoids, acetogenins, amino-acid derivates and polyphenols). Many of these compounds can act as antimicrobial and [[antifouling agent|antifouling]] or ultraviolet screening agents, as well as herbivore deterrents. | + | [[Diversity and classification of marine benthic algae|Seaweeds]] have been shown to produce a large variety of metabolites with highly variable structures (such as terpenoids, acetogenins, amino-acid derivates and polyphenols). Many of these compounds can act as antimicrobial and [[antifouling agent|antifouling]] or ultraviolet screening agents, as well as herbivore deterrents. |
Most marine herbivores are generalist grazers that consume many different seaweeds, although some herbivore species can be specialised on one or a few algal species. | Most marine herbivores are generalist grazers that consume many different seaweeds, although some herbivore species can be specialised on one or a few algal species. | ||
| − | Grazing pressure highly depends on the specific seaweed and herbivore involved. Grazing pressure is however generally considered be higher in tropical coral reefs than in temperate habitats. Large mobile grazers, such as fish, crabs and sea urchins, can have a more drastic negative effect on seaweed production and fitness than smaller ones. Due to their ability to rapidly consume large amounts of algal tissues, they are thought to select for constitutive defences (i.e., defences which are continuously produced and present within the algae). | + | Grazing pressure highly depends on the specific seaweed and herbivore involved. Grazing pressure is however generally considered to be higher in tropical [[coral reefs]] than in temperate habitats. Large mobile grazers, such as fish, crabs and sea urchins, can have a more drastic negative effect on seaweed production and fitness than smaller ones. Due to their ability to rapidly consume large amounts of algal tissues, they are thought to select for constitutive defences (i.e., defences which are continuously produced and present within the algae). |
| − | Smaller grazers use plants both as food and habitat, and they consume individual algae over a more extended period of time. It has been hypothesised that smaller grazers may select for inducible rather than constitutive defences (i.e., | + | Smaller grazers use plants both as food and habitat, and they consume individual algae over a more extended period of time. It has been hypothesised that smaller grazers may select for inducible rather than constitutive defences (i.e., defense mechanisms produced in response to specific environmental signals). |
| + | <P> | ||
| + | <BR> | ||
| + | ===Chemical ecology and animals=== | ||
The hypothesis that sessile or slow-moving organisms, without obvious escape mechanisms | The hypothesis that sessile or slow-moving organisms, without obvious escape mechanisms | ||
and physical protection, are more likely to defended themselves chemically has been explored in the marine environment. Of these organisms, | and physical protection, are more likely to defended themselves chemically has been explored in the marine environment. Of these organisms, | ||
| − | [http://www.marinespecies.org/aphia.php?p=taxdetails&id=382226 opisthobranch] | + | [http://www.marinespecies.org/aphia.php?p=taxdetails&id=382226 opisthobranch molluscs] appear to be particularly well endowed with secondary metabolites. These gastropods compensate the reduction of their shells by the development of complex defence strategies that include use of chemicals. Opisthobranchs can feed |
upon [http://www.marinespecies.org/aphia.php?p=taxdetails&id=558 sponges], algae, [http://www.marinespecies.org/aphia.php?p=taxdetails&id=19494 hydroids], [http://www.marinespecies.org/aphia.php?p=taxdetails&id=146142 bryozoans], [http://www.marinespecies.org/aphia.php?p=taxdetails&id=146420 tunicates] and soft corals. In some cases they are not only capable to accumulate dietary molecules but can also transform or even produce new chemical mediators (see D on figure). | upon [http://www.marinespecies.org/aphia.php?p=taxdetails&id=558 sponges], algae, [http://www.marinespecies.org/aphia.php?p=taxdetails&id=19494 hydroids], [http://www.marinespecies.org/aphia.php?p=taxdetails&id=146142 bryozoans], [http://www.marinespecies.org/aphia.php?p=taxdetails&id=146420 tunicates] and soft corals. In some cases they are not only capable to accumulate dietary molecules but can also transform or even produce new chemical mediators (see D on figure). | ||
| − | [http://www.marinespecies.org/aphia.php?p=taxdetails&id=140672 ''Oxynoe olivacea''], a green sea snail that lives camouflaged upon algae ([http://www.marinespecies.org/aphia.php?p=taxdetails&id=143816 ''Caulerpa'']), is able to transform a major algal metabolite, caulerpenyne, to oxytoxins, which are 100 times more toxic (see E on figure) | + | [http://www.marinespecies.org/aphia.php?p=taxdetails&id=140672 ''Oxynoe olivacea''], a green sea snail that lives camouflaged upon algae ([http://www.marinespecies.org/aphia.php?p=taxdetails&id=143816 ''Caulerpa'']), is able to transform a major algal metabolite, caulerpenyne, to oxytoxins, which are 100 times more toxic (see E on figure)<ref name="ma"/>. |
<P> | <P> | ||
<BR> | <BR> | ||
| Line 66: | Line 70: | ||
== See also == | == See also == | ||
*[[Chemical and physical properties of functional metabolites]] | *[[Chemical and physical properties of functional metabolites]] | ||
| − | *[[Marine Functional Metabolites]] | + | *[[Marine Functional Metabolites|Functional Metabolites]] |
| − | *[[Functional metabolites | + | **[[Chemical and physical properties of functional metabolites|Chemical and physical properties]] |
| − | *[[Functional metabolites in phytoplankton]] | + | **[[Functional metabolites and macroalgal-herbivore interactions|Macroalgal-herbivore interactions]] |
| − | *[[Functional metabolites | + | **[[Functional metabolites in phytoplankton|Phytoplankton]] |
| + | **[[Functional metabolites in benthic invertebrates|Benthic invertebrates]] | ||
<P> | <P> | ||
<BR> | <BR> | ||
| Line 76: | Line 81: | ||
==References== | ==References== | ||
<references/> | <references/> | ||
| + | |||
| + | [[Category: MarBEF Wiki]] | ||
| + | [[Category: Chemical ecology]] | ||
Latest revision as of 15:17, 27 August 2023
Contents
The importance of chemical ecology
Chemical ecology has helped to understand terrestrial ecosystems. How bees pollinate flowers, how birds find their nests and human attractiveness to a partner are some of the many examples of interactions which are mediated by chemicals. It is not difficult to imagine the catastrophic consequences of the absence of such crucial relationships. Imagine a similar scenario without chemical interactions in the marine environment. Species would no longer be able to identify their food, locate their pray, recognize mates,... . Species-specific chemicals can shape community processes such as seasonal succession, niche structure, selective feeding and population dynamics.
The MarBEF ROSEMEB (Role of Secondary Metabolites in Ecosystem Biodiversity) project has provided a better understanding of the roles of these chemicals in maintaining marine biodiversity and driving ecosystem functionality. Some of these findings are discussed bellow[1].
Chemical ecology and microbes
Microbes sense their environment via cell-associated and diffusible molecules such as AHL (N-acylhomoserine lactones). Such molecules are constantly produced by many bacteria and diffuse through membranes into the surrounding environment.
When a certain cell density (a threshold or quorum) of the bacterial population and a corresponding concentration of AHL is reached, the expression of certain target genes is initiated. The proteins expressed by these genes may include the proteins for light emission in luminous bacteria or pathogenic factors that cause disease.
This quorum-sensing typically controls processes, such as swarming (coordinated movement), virulence (coordinated attack) or conjugation (gene transfer between cells), which require high cell densities for success and that are essential for the survival of the organisms which produce the molecules[1].
Chemical ecology and phytoplankton
Many plankton species use a chemical defence against their predators, either through toxin production or feeding deterrence. Diatoms are key players at the base of the marine food web and have always been assumed to be a good food source for herbivores. Some species however use chemicals as a defence against being grazed. The discovery that these unicellular algae produced chemicals, such as polyunsaturated aldehydes (PUAs) and other oxidised products of fatty acid metabolism (collectively termed oxylipins), that induced abortions, birth defects, poor development and high offspring mortality to their grazers has changed our view of plant-animal interactions in the plankton. (C on the figure)
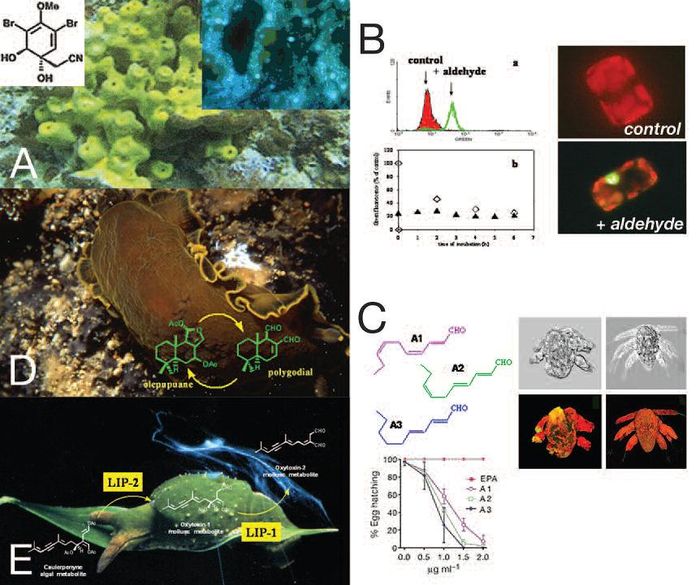
Although these toxins don't cause death, they can have ecological effects. They can sabotage future generations of grazers and therefore allow diatom blooms to persist when grazing pressure would normally have caused them to crash. This defence mechanism is new and specific for the marine environment. In contrast, most of the known (terrestrial) negative plant–animal interactions are related to poisoning, repellence or feeding deterrence instead of to reproductive failure.
In fact, the production of PUAs mainly affects future generations of grazers and have less effects on the direct adult grazers. PUAs have also been shown to negatively impact other phytoplankton cells where they might function as a signal to trigger active cell-death. (B on the figure)
So, these compounds may have multiple functions within plankton communities. They can act as defence molecules against predators and competitors, as well as signal molecules to drive diatom bloom dynamics and species succession patterns. More information can be found here.
Other phytoplankton groups such as the dinoflagellates produce neurotoxins that can be transferred (biomagnify) up the marine food chain and have been responsible for mass fish-kills, as well as for the deaths of sea birds and marine mammals, including whales and sea lions. See also here.
In humans, consumption of shellfish containing high levels of such toxins can induce paralytic, neurotoxic, diarrhetic and amnesic shellfish poisoning. Records of human poisoning by at least two of these syndromes date back hundreds of years, yet the discovery and characterisation of the responsible molecules happened quite recent.
Many benthic invertebrates can use compounds from the food they consume, as defensive molecules against predators. Some plankton species might possibly do the same. Lots of research still needs to be conducted on the effects of toxins on gamete, embryonic and larval development of herbivorous grazers[1].
Chemical ecology and seaweeds
Seaweeds have been shown to produce a large variety of metabolites with highly variable structures (such as terpenoids, acetogenins, amino-acid derivates and polyphenols). Many of these compounds can act as antimicrobial and antifouling or ultraviolet screening agents, as well as herbivore deterrents.
Most marine herbivores are generalist grazers that consume many different seaweeds, although some herbivore species can be specialised on one or a few algal species. Grazing pressure highly depends on the specific seaweed and herbivore involved. Grazing pressure is however generally considered to be higher in tropical coral reefs than in temperate habitats. Large mobile grazers, such as fish, crabs and sea urchins, can have a more drastic negative effect on seaweed production and fitness than smaller ones. Due to their ability to rapidly consume large amounts of algal tissues, they are thought to select for constitutive defences (i.e., defences which are continuously produced and present within the algae).
Smaller grazers use plants both as food and habitat, and they consume individual algae over a more extended period of time. It has been hypothesised that smaller grazers may select for inducible rather than constitutive defences (i.e., defense mechanisms produced in response to specific environmental signals).
Chemical ecology and animals
The hypothesis that sessile or slow-moving organisms, without obvious escape mechanisms and physical protection, are more likely to defended themselves chemically has been explored in the marine environment. Of these organisms, opisthobranch molluscs appear to be particularly well endowed with secondary metabolites. These gastropods compensate the reduction of their shells by the development of complex defence strategies that include use of chemicals. Opisthobranchs can feed upon sponges, algae, hydroids, bryozoans, tunicates and soft corals. In some cases they are not only capable to accumulate dietary molecules but can also transform or even produce new chemical mediators (see D on figure).
Oxynoe olivacea, a green sea snail that lives camouflaged upon algae (Caulerpa), is able to transform a major algal metabolite, caulerpenyne, to oxytoxins, which are 100 times more toxic (see E on figure)[1].
See also
References
- ↑ 1.0 1.1 1.2 1.3 Heip, C., Hummel, H., van Avesaath, P., Appeltans, W., Arvanitidis, C., Aspden, R., Austen, M., Boero, F., Bouma, TJ., Boxshall, G., Buchholz, F., Crowe, T., Delaney, A., Deprez, T., Emblow, C., Feral, JP., Gasol, JM., Gooday, A., Harder, J., Ianora, A., Kraberg, A., Mackenzie, B., Ojaveer, H., Paterson, D., Rumohr, H., Schiedek, D., Sokolowski, A., Somerfield, P., Sousa Pinto, I., Vincx, M., Węsławski, JM., Nash, R. (2009). Marine Biodiversity and Ecosystem Functioning. Printbase, Dublin, Ireland ISSN 2009-2539