Trichloromethane
Definition of trichloromethane:
Trichloromethane, better known as chloroform, is a member of the group of trihalomethanes. It is a colourless liquid with a sweet taste and odour. Breathing large amounts of its vapour will cause headaches, sleepiness and unconsciousness[1].
This is the common definition for trichloromethane, other definitions can be discussed in the article
|
Notes
| Trichloromethane |
|---|
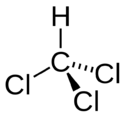
|
| Formula |
| CHC3 |
Chloroform is used mainly as a raw material in the production of hydrochlorofluorocarbon-22, which will be phased out in the European Union by 2025. Chloroform is used in other applications including in the pharmaceutical industry (for example in the extraction of penicillin and other antibiotics). It is also used as a degreasing agent and as a chemical intermediate in the production of dyes, pesticides and other substances. Chloroform is produced in the European Union at a yearly volume of 302.800 tons (in 2002). Of this an estimated 4000 tons are released into the environment (2000 tons by evaporation to the atmosphere and 2000 tons in waste waters), both from production sites and from use products derived from it. It has been suggested that chloroform might be released through natural processes, although this still remains to be proven[2].
Chloroform is a volatile substance causing it to evaporate rapidly into the atmosphere. There it is quite stable with a half-life of up to 100 days. It's even more stable in water bodies, where there is little abiotic degradation and also not much biodegradation. Chloroform has a moderate water solubility of 8 g/l and no high tendency towards adsorption to soils or sediments[2].
Chloroform has only a low potential to bioaccumulate in fishes, although moderate bioaccumulation may occur in small aquatic species. It is not expected to significantly biomagnify through food chains.
Short exposure to chloroform concentrations of 20 mg/l can be lethal to some fish and zooplankton species, although most can tolerate short exposure to concentrations up to 100 mg/l. Lower concentrations can be toxic at continuous exposure.
Chloroform concentrations measured in the open ocean range between 0,0016 µg/l and 1 µg/l. In the Scheldt estuary concentrations of 0,15 µg/l have been measured. Heavily polluted coastal waters may contain concentrations up to 70µg/l[2].
Environmental standards and legislation
Included in the water framework list of priority substances
See also
Chloroform on the ED North Database
Chloroform on the Ecotox Database
References
Please note that others may also have edited the contents of this article.
|