Hexachlorobenzene
Definition of hexachlorobenzene (HCB):
Hexachlorobenzene is a white crystalline solid which doesn't occur naturally. Hexachlorobenzene was widely used as a pesticide until 1965. It was also used to make fireworks, ammunition, and synthetic rubber[1].
This is the common definition for hexachlorobenzene (HCB), other definitions can be discussed in the article
|
Notes
| Hexachlorobenzene |
|---|
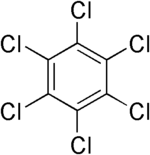
|
| Formula |
| C6Cl6 |
Due to environmental concerns, the use of HCB in such applications has now virtually ceased in Europe and the US, although it may still be used in some other parts of the world. However releases still occur and prolong the presence of HCB in the environment. HCB is also formed as a by-product during the manufacture of chemicals used as solvents, other chlorine-containing compounds, and pesticides. Small amounts of hexachlorobenzene can be produced during combustion processes such as burning of city wastes. European emissions in 1997 were 100 kg in waste waters and 4 kg into the atmosphere. World wide emissions in 1995 still ranged between 12.000 and 95.000 kg[2].
Hexachlorobenzene is one of the more persistent environmental pollutants because of its chemical stability and resistance to biodegradation. In the atmosphere of temperate regions it has a half-life of 1,94 years, in atmosphere of polar regions it has one of 6,28 years. This high persistence makes long-range global transport very likely. From the atmosphere HCB can washed out by rainfall or snowfall, or removed via dry deposition. In water HCB has a very low water solubility of only 6 µg/l, and most of it is adsorbed to suspended particulate matter and sediments. It is also very persistent in water and soils with a half-life of 2,7 and 6 years respectively. Although HCB is moderately volatile, its strong adsorption to particles prolongs its present in the marine environment[1].
Hexachlorobenzene shows very high bioaccumulation rates. Lichens have been reported to bioaccumulate, by direct adsorption, 17 million times the surrounding environmental concentration, fishes 21.900 times[1]. The high trophic level marine mammals or sea birds can metabolise and excrete HCB and therefore don't seem to bioaccumulate it[2].
Concentrations up to the maximum of solubility (5 µg/l) showed no adverse effects on marine fishes. Some species of marine invertebrates are affected by concentrations above 20 µg/l. Through biomagnification it poses a threat of secondary poisoning to birds and mammals. It has been suggested that prolonged exposure to a daily digestion above 80 µg per kilogram of body weight might cause cancer in mammals. Birds which were fed with food containing more than 5 mg/kg HCB, began developing liver abnormalities after a period of 90 days[2].
The concentrations measured in European marine waters range from 0.001 ng/l to 196 ng/l (Forth estuary, Scotland in 1987). Recent measurements suggest that most marine and estuarine concentrations are bellow 1 ng/l. Concentrations in the livers of marine fish have been shown to range between 4 to 570 µg/kg wet weight. Concentrations in the edible parts are however much lower. HCB concentrations in fishes are decreasing and a study from 1995 showed them to range between 10 and 25 µg/kg lipid weight[2].
Environmental standards and legislation
Included in the water framework list of priority substances
See also
Hexachlorobenzene on ED North Database
References
Please note that others may also have edited the contents of this article.
|