Glyphosate
Definition of glyphosate:
Glyphosate was first used as a herbicide in 1973. It is among the worlds most widely used herbicides and is used in 130 countries for the weed control of more than 100 crops. [1] It occurs as a white crystalline solid. [2]
This is the common definition for glyphosate, other definitions can be discussed in the article
|
Notes
| Glyphosate |
|---|
|
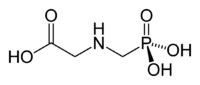
| Formula |
| C3H8NO5P |
The glyphosate is used as a herbicide to control a number of broadleaf weeds and grasses. The principal food use sites include corn, wheat, sorghum, citrus and stone fruits, potatoes and onions, asparagus, coffee, peanuts, and pineapples. There are also a number of non-food use sites including ornamental, turf, forestry, and industrial rights-of-way (rail road tracks).[2]
Although it is rather soluble in water (11,6 g/l), in application sites, glyphosate adsorbs to soils and should stay in the top 15 cm. This reduces its exposure to surface waters and the marine environment. Glyphosate is a rather unstable molecule that can be biodegraded. In most environments, its half-life is less than 30 days, although in some cases it takes up to 174 days to half its environmental concentration. Glyphosate is usually biodegraded to AMPA.[2]
Glyphosate doesn't have a tendency to bioaccumulate or biomagnify.[2]
Concentrations of 10µg/l might cause acute toxicity in one water flea species, while other zooplankton can tolerate short exposure to glyphosate concentrations of 25 mg/l. Concentrations which cause acute toxicity in fish range from 5 mg/l to 19 g/l, depending on the species. [3]
Concentrations in fresh surface water range between 0.5 and 1700 µg/l. [4]
Environmental standards and legislation
Included in the water framework list of priority substances
See also
Glyphosate on ED North Database