Glyphosate
Definition of glyphosate:
Glyphosate was first used as a herbicide in 1973. Today it's used in Roundup WeatherMax, Roundup UltraMax and other non selective herbicides. It is among the worlds most widely used herbicides, is used in 130 countries for weed control of more than 100 crops. [1] It occurs as a white crystalline solid. [2]
This is the common definition for glyphosate, other definitions can be discussed in the article
|
Notes
| Glyphosate |
|---|
|
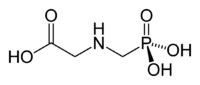
| Formula |
| C3H8NO5P |
The glyphosate is used as a herbicide to control a number of broadleaf weeds and grasses. The principal food use sites include corn, wheat, sorghum, citrus and stone fruits, potatoes and onions, asparagus, coffee, peanuts, and pineapples. There are also a number of non-food use sites including ornamental, turf, forestry, and industrial rights-of-way (rail road tracks).[2]
Although it is rather soluble in water (11,6 g/l), in application sites, glyphosate adsorbs to soils and should stay in the top 15 cm of soils. This reduces its exposure to surface waters and the marine environment. It is a rather unstable molecule that can be biodegraded. In most environments, it usually takes less than 30 days to halve its concentration, although it can in some cases take up to 174 days. Glyphosate is usually biodegraded to AMPA.[2]
Glyphosate doesn't have a tendency to bioaccumulate or biomagnify.[2]
Concentrations of 10µg/l might cause acute toxicity in one water flea species, while other zooplankton can tolerate concentrations up to or above 25 mg/l. Some fish species start dying at concentrations above 5 mg/l while others like goldfish might even tolerate concentrations above 19 g/l. [3]
Concentrations in fresh surface water range between 0.5 and 1700 µg/l. [4]
Environmental standards and legislation
Included in the water framework list of priority substances
See also
Glyphosate on ED North Database