Trichloromethane
Definition of trichloromethane:
Trichloromethane better known as chloroform is a member of the group of trihalomethanes. It is a colourless liquid with a sweet taste and odour. Breathing large amounts of its vapour will cause headaches, sleepiness and unconsciousness. Chloroform boils at 63°C. [1]
This is the common definition for trichloromethane, other definitions can be discussed in the article
|
Notes
| Trichloromethane |
|---|
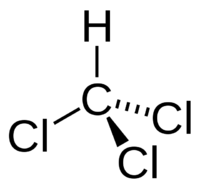
|
| Formula |
| CHC3 |
Chloroform is used mainly as a raw material in the production of hydrochlorofluorocarbon-22, which will be phased out in the European Union by 2025. Chloroform is used in other applications including in the pharmaceutical industry (for example in the extraction of penicillin and other antibiotics). It is also used as a degreasing agent and as a chemical intermediate in the production of dyes, pesticides and other substances. Chloroform is produced in the European Union at a yearly volume of 302.800 tons (in 2002). Of this an estimated 4000 tons (2000 tons by evaporation to the atmosphere and 2000tons in waste waters) are released into the environment, both from production sites and by use products derived from it. It has been suggested that chloroform might be released through natural processes, although this still remains to be proven. [2]
Chloroform is a volatile substance causing it to evaporate easily into the atmosphere. There it is quite stable as it takes up to 100 days to half its concentration. It's even more stable in water bodies, where there is little abiotic degradation and also not much biodegradation. It has a moderate water solubility of 8 g/l. It doesn't have a high tendency towards adsorption to soils or sediments. [2]
Chloroform has only a low potential to bioaccumulate in fishes, although moderate bioaccumulation may occur in small aquatic species. It is not expected to significantly biomagnify through food chains.
Some fish and zooplankton species species die at concentrations above 20 mg/l although most can tolerate concentrations up to 100 mg/l. Chronic exposure can lead to deaths at lower concentrations.
Concentrations measured in the open ocean range between 0,0016 µg/l and 1 µg/l. In the Scheldt estuary concentrations of 0,15 µg/l have been measured. Heavily polluted coastal waters may contain concentrations up to 70µg/l.[2]
Environmental standards and legislation
Included in the water framework list of priority substances
See also
Chloroform on ED North Database