Anthracene
| Anthracene |
|---|
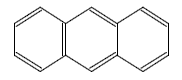
|
| Formula |
| C14H10 |
Natural state and human emission
Anthracene is used as an intermediate compound for the manufacturing of dyes and polyradicals used to make resins. It may also be used as a diluent for wood protection products, an insecticide, or a fungicide. Furthermore, anthracene is an organic cristallized photoconductor used in electrophotography.
In the nature, anthracene can be found to a small degree in fossil fuels (12 g/kg of coal). It is also present in fuel (100 to 300 mg/L) and petrol (1.55 mg/L, to 2,6 mg/L in high-octane petrols)[1].
The main sources of anthropic emissions are the following:
- tailpipes of car motors (0.02 to 6.45 μg/m3)[2];
- cokefaction and gaseifaction of coal and more generally emissions of coal kilns and fuel kilns;
- purification of petrol;
- use of impregnation oils for wood treatment;
- production of asphalt for roads covering;
- smoke of wood coal;
- combustion of pneumatic wastes (rubber).
Behaviour
In water, anthracene is easily adsorbed on particles in suspension. However, it may also volatilize in the same time, which means its final amount is the result of two processes in competition. In soils, anthracene does not move very much: it may volatilizy on moist soils, but only very little on dry soils.[3]
In distilled water, anthracene is destroyed by photolysis in several hours when it is exposed to natural light. However, the substance is not biodegradable: after 28 days in living organisms, only 1.9% of the substance is destroyed.[4]
Anthracene may also accumulate in aquatic organisms, with a ratio of absorption speed to elimination speed equal to 10,000 for some organisms.[5][6][7]
Effects on health
References
- ↑ Verschueren K. (1996) - Anthracene. Handbook of Environmental Data on Organic Chemicals. New York, Van Nostrand Reinhold Co. 3rd Ed, pp. 214-217
- ↑ OMS IPCS (1998) - Environmental Health Criteria 202: Selected Non-Heterocyclic Policyclic Aromatic Hydrocarbons. World Health Organisation, International Programme on Chemical Safety. http://www.inchem.org/fullist.htm
- ↑ HSDB (2001) - Polycyclic Aromatic Hydrocarbons - Anthracene. Hazardous Substances Data Bank, National Library of Medicine. http://www.toxnet.nlm.nih.gov
- ↑ Callahan M.A., Slimak M.W., Gabel N.W., May I.P., Fowler C.F., Freed J.R., Jennings P., Durfee R.L., Whitmore F.C., Maestri W.R., Mabey B.R. and Holt B.R. (1979) - Water-related environmental fate of 129 priority pollutants Volume II. Halogenated aliphatic hydrocarbons, halogenated ethers, monocyclic aromatics, phtalates esters, polycyclic aromatic hydrocarbons, nitrosamines, miscellaneous compounds. United States Environmental Protection Agency. Washington, DC. EPA-440/4-79-029b.
- ↑ Spacie A., Landrum P. and Leversee G. (1983) - Uptake, depuration, and biotransformation of anthracene and benzo[a]pyrene in bluegill sunfish. Ecotoxicol Environ Saf, 7, 330-341.
- ↑ Linder G., Bergman H. and Meyer J. (1985) - Anthracene bioconcentration in rainbow-trout during single-compound and complex-mixture exposures. Environ Toxicol Chem, 4, 549-558.
- ↑ CITI (1992) - Biodegradation and Bioaccumulation data of existing chemicals based on the CSCL Japan. Chemicals Inspection and Testing Institute. Japan. October 1992.