Anthracene
From Coastal Wiki
| Anthracene |
|---|
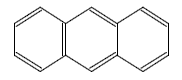
|
| Formula |
| C14H10 |
Natural state and human emission
Anthracene is used as an intermediate compound for the manufacturing of dyes and polyradicals used to make resins. It may also be used as a diluent for wood protection products, an insecticide, or a fungicide. Furthermore, anthracene is an organic cristallized photoconductor used in electrophotography.
In the nature, anthracene can be found to a small degree in fossil fuels (12 g/kg of coal). It is also present in fuel (100 to 300 mg/L) and petrol (1.55 mg/L, to 2,6 mg/L in high-octane petrols).
The main sources of anthropic emissions are the following:
- tailpipes of car motors (0.02 to 6.45 μg/m3);
- cokefaction and gaseifaction of coal and more generally emissions of coal kilns and fuel kilns;
- purification of petrol;
- use of impregnation oils for wood treatment;
- production of asphalt for roads covering;
- smoke of wood coal;
- combustion of pneumatic wastes (rubber).