Bisphenol-A
Definition of Bisphenol-A:
Bisphenol-A is also known as 4,4’-Isopropylidenediphenol. At room temperature it occurs as a white powder or in flakes[1].
This is the common definition for Bisphenol-A, other definitions can be discussed in the article
|
Notes
| Bisphenol-A |
|---|
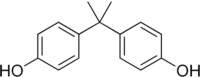
|
| Formula |
| C15H16O2 |
The total amount of bisphenol-A manufactured in 2005 within the EU was estimated at approximately 1.150.000 tonnes. This large amount was mainly produced to manufacture polycarbonate, witch is a widely used plastic[2].
Bisphenol A is moderately soluble in water (300 mg/l), it is considered to have a moderate tendency to adsorb to suspended particles and sediments. It has a very low tendency to evaporate into the atmosphere where most of it will be degraded in less than a day. In water and soils it is chemically stable, although it can readily be biodegraded. The environmental half-life is only 3 to 8 days and a 100% removal of environmental contamination can occur within 17 days. In anoxic sediments bisphenol A can be created from the degradation of TBBP-A[1].
Bisphenol A has a low tendency to bioaccumulate. Therefore it poses a low toxicity threat by biomagnification towards marine mammals. Bisphenol A becomes lethal when mammals consume each day more than 33 mg of it per kg of body weight[1].
Bisphenol A exhibits endocrine disrupting effects. In gastropods concentrations bellow 100 µg/l have been shown to cause reduced penis sizes in males and enhanced oocyte production in females. The latter results in an increased embryo production at low bisphenol A concentrations. This effect has even been demonstrated in some gastropod species at concentrations of only 100 ng/l[2]. It demonstrates a moderate acute toxicity towards aquatic species as most species tolerate short exposure to concentrations of 1 mg/l[3].
Environmental standards and legislation
Included in the water framework list of priority substances
See also
Bisphenol-A on the ED North Database
Bisphenol-A on the Ecotox Database