Naphthalene
Definition of naphthalene:
Naphthalene is a white solid that evaporates easily. Fuels such as petroleum and coal contain naphthalene. It is also called white tar and tar camphor. It has been used in mothballs and moth flakes. Burning tobacco or wood produces naphthalene. It has a strong but not unpleasant smell. [1]
This is the common definition for naphthalene, other definitions can be discussed in the article
|
Notes
| Naphthalene |
|---|
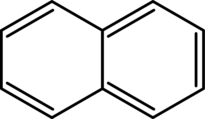
|
| Formula |
| C10H8 |
Naphthalene can be produced from coal or from petroleum. Production volume in the United States decreased significantly from a peak of 409.000 tons in 1968 to 101.000 tons in 1994. Production capacity has remained relatively stable in recent years, with estimated capacity for 2004 at 97,700 tons. The major commercial use of naphthalene is in the manufacture of polyvinyl chloride (PVC) plastics. The major consumer products made from naphthalene are moth repellents, in the form of mothballs or crystals, and toilet deodorant blocks. It is also used for making dyes, resins, leather tanning agents, and the insecticide carbaryl. It enters the environment from industrial uses, from its use as a moth repellent, from the burning of wood or tobacco and from accidental spills. [1]
Most of the naphthalene entering the environment is discharged to the air. The largest releases result from the combustion of wood and fossil fuels and the off-gassing of naphthalene-containing moth repellents. Smaller amounts of naphthalene are introduced to water as the result of discharges from coaltar production and distillation processes. The coal-tar industry is also a major source of the small amounts of naphthalene that are directly discharged to land.
In the atmosphere naphthalene is broken down rapidly, usually within one day. From the atmosphere it can also very slowly be depositioned in water. It has a rather low water solubility of 31,7 µg/l and a rather low tendency to adsorb to particles. It is expected that only 10% of the naphthalene in water bodies is associated with particles. The main loss of naphthalene from water water bodies is due to evaporate into the atmosphere.
Naphthalene has a very low potential towards bioaccumulation, and will be rapidly eliminated from vertebrates. Therefore naphtalene is not expected to biomagnify through food chains.[1]
Concentrations above 1 mg/l can be toxic for some species of fish, crustaceans and zooplankton. [2]
Naphthalene concentrations in the South Atlantic ocean are around 6,3 ng/l.[1]
Environmental standards and legislation
Included in the water framework list of priority substances
See also
Naphthalene on the ED North Database