Difference between revisions of "Mariculture"
Dronkers J (talk | contribs) |
Dronkers J (talk | contribs) |
||
| Line 1: | Line 1: | ||
| + | |||
__NOTOC__ | __NOTOC__ | ||
| Line 4: | Line 5: | ||
Mariculture is often defined as aquaculture in marine environments. | Mariculture is often defined as aquaculture in marine environments. | ||
Some limit mariculture to culture of marine plants and animals in the ocean itself (EEA, 2008<ref>European Environmental agency; https://www.eea.europa.eu/help/glossary/eea-glossary/mariculture</ref>). | Some limit mariculture to culture of marine plants and animals in the ocean itself (EEA, 2008<ref>European Environmental agency; https://www.eea.europa.eu/help/glossary/eea-glossary/mariculture</ref>). | ||
| − | Others also include species from brackish water and include culture methods that take place in salty and brackish water that is | + | Others also include species from brackish water and include culture methods that take place in salty and brackish water that is situated in the coastal zone (CBD, 2004<ref name="CBD">Secretariat of the Convention on Biological Diversity (2004): Solutions for sustainable mariculture-avoiding the adverse effects of mariculture on biological diversity, CBD Technical Series No. 12</ref>; Wecker, 2006<ref name="wecker">Wecker B (2006): Nährstofffluss in einer geschlossenen Kreislaufanlage mit integrierter Prozesswasserklärung über Algenfilter-Modell und Wirklichkeit.; https://macau.uni-kiel.de/receive/dissertation_diss_00001878</ref>). Here this wider definition is referred to. |
Mariculture can be distinguished from capture fisheries by two criteria: ownership of the stock and deliberate intervention in the production cycle (husbandry) (Naylor et al., 2000<ref name="naylor">Naylor RL, Goldburg RJ, Primavera JH, Kautsky N, Beveridge MCM, Clay J, Folke C, Lubchenko J, Mooney H, Troell M (2000): Effect of aquaculture on world fish supplies; Nature 405, p. 1017-1024</ref>). | Mariculture can be distinguished from capture fisheries by two criteria: ownership of the stock and deliberate intervention in the production cycle (husbandry) (Naylor et al., 2000<ref name="naylor">Naylor RL, Goldburg RJ, Primavera JH, Kautsky N, Beveridge MCM, Clay J, Folke C, Lubchenko J, Mooney H, Troell M (2000): Effect of aquaculture on world fish supplies; Nature 405, p. 1017-1024</ref>). | ||
==Introduction== | ==Introduction== | ||
| − | [[File: | + | [[File:WorldProdFisheryAquacultureFAO2020.jpg|thumb|left|500px|Fig. 1. Global trends in different types of fisheries, showing the fast increase of aquaculture. In 2020 the total aquaculture production (algae mariculture excluded) exceeded the production of capture (wild) fisheries. The production of inland aquaculture was about twice the production of mariculture. Source: FAO (2022<ref name=FAO>FAO (2022) The State of World Fisheries and Aquaculture 2022. Towards Blue transformation. Rome. https://doi.org/10.4060/cc0461en </ref>). Creative Commons Licence.]] |
| − | Mariculture includes a wide range of species and culture methods. | + | Mariculture includes a wide range of species and culture methods. It is globally a fast-growing activity, see Fig. 1. This is due to the fact that many wild fish stocks are overfished and catches are declining (Wecker, 2006<ref name="wecker"/>). |
| − | It is globally a fast-growing activity | ||
| − | This is due to the fact that many wild fish stocks are overfished and catches are declining ( | ||
At the same time the world population is rising and with it the need for dietary protein. | At the same time the world population is rising and with it the need for dietary protein. | ||
The expansion of mariculture can reduce pressure on wild fish, shrimps and molluscs, because they reduce their market price and by this the investments in fishing fleets. However, they can also increase the pressure due to the use of fishmeal in feed for some mariculture-species (Naylor et al., 2000<ref name="naylor">Naylor RL, Goldburg RJ, Primavera JH, Kautsky N, Beveridge MCM, Clay J, Folke C, Lubchenko J, Mooney H, Troell M (2000): Effect of aquaculture on world fish supplies; Nature 405, p. 1017-1024</ref>). | The expansion of mariculture can reduce pressure on wild fish, shrimps and molluscs, because they reduce their market price and by this the investments in fishing fleets. However, they can also increase the pressure due to the use of fishmeal in feed for some mariculture-species (Naylor et al., 2000<ref name="naylor">Naylor RL, Goldburg RJ, Primavera JH, Kautsky N, Beveridge MCM, Clay J, Folke C, Lubchenko J, Mooney H, Troell M (2000): Effect of aquaculture on world fish supplies; Nature 405, p. 1017-1024</ref>). | ||
| − | Some forms of mariculture provide good quality food and the production is more efficient than that of terrestrial animals; roughly half the level of feed input per unit output is necessary (CBD, 2004<ref name="CBD"/>). | + | Some forms of mariculture provide good quality food and the production is more efficient than that of terrestrial animals; roughly half the level of feed input per unit output is necessary (CBD, 2004<ref name="CBD"/>). Products obtained from mariculture are not only used for food, but also as raw material for e.g. cosmetics, neutraceuticals, medicines, food additives and many more. |
| − | + | ||
| − | + | The main species grown in mariculture are listed in Table 1. The countries with the largest production are listed in Table 2. Most mariculture is concentrated in East Asia. | |
| − | + | ||
| − | Products obtained from mariculture are not only used for food, but also as raw material for e.g. cosmetics, neutraceuticals, medicines, food additives and many more. | + | |
| + | {| style="border-collapse:collapse; font-size: 12px; background:ivory;" cellpadding=5px align=center width=100% | ||
| + | |+ Table 1. Major mariculture species in 2020<ref name=FAO>FAO (2022) The State of World Fisheries and Aquaculture 2022. Towards Blue transformation. Rome. https://doi.org/10.4060/cc0461en </ref>. The figures include coastal aquaculture. | ||
| + | |- style="font-weight:bold; font-size: 10px; text-align:center; background:lightblue" | ||
| + | ! width="20% style="border:2px solid blue;"| Finfish | ||
| + | ! width="5% style="border:2px solid blue;"| thousand | ||
| + | tonnes | ||
| + | ! width="20% style="border:2px solid blue;"| Crustaceans | ||
| + | ! width="5% style="border:2px solid blue;"| thousand | ||
| + | tonnes | ||
| + | ! width="20% style="border:2px solid blue;"| Molluscs | ||
| + | ! width="5% style="border:2px solid blue;"| thousand | ||
| + | tonnes | ||
| + | ! width="20% style="border:2px solid blue;"| Seaweed | ||
| + | ! width="5% style="border:2px solid blue;"| thousand | ||
| + | tonnes | ||
| + | |- | ||
| + | | style="border:2px solid lightblue; font-size: 10px; text-align:center"| Atlantic salmon | ||
| + | | style="border:2px solid lightblue; font-size: 10px; text-align:center"| 2720 | ||
| + | | style="border:2px solid lightblue; font-size: 10px; text-align:center"| Whiteleg shrimp | ||
| + | | style="border:2px solid lightblue; font-size: 10px; text-align:center"| 5812 | ||
| + | | style="border:2px solid lightblue; font-size: 10px; text-align:center"| Cupped oyster | ||
| + | | style="border:2px solid lightblue; font-size: 10px; text-align:center"| 5450 | ||
| + | | style="border:2px solid lightblue; font-size: 10px; text-align:center"| Japanese kelp | ||
| + | | style="border:2px solid lightblue; font-size: 10px; text-align:center"| 12470 | ||
| + | |- | ||
| + | | style="border:2px solid lightblue; font-size: 10px; text-align:center"| Milkfish | ||
| + | | style="border:2px solid lightblue; font-size: 10px; text-align:center"| 1168 | ||
| + | | style="border:2px solid lightblue; font-size: 10px; text-align:center"| Chinese mitten crab | ||
| + | | style="border:2px solid lightblue; font-size: 10px; text-align:center"| 776 | ||
| + | | style="border:2px solid lightblue; font-size: 10px; text-align:center"| Japanese carpet shell | ||
| + | | style="border:2px solid lightblue; font-size: 10px; text-align:center"| 4266 | ||
| + | | style="border:2px solid lightblue; font-size: 10px; text-align:center"| Eucheuma seaweeds | ||
| + | | style="border:2px solid lightblue; font-size: 10px; text-align:center"| 8129 | ||
| + | |- | ||
| + | | style="border:2px solid lightblue; font-size: 10px; text-align:center"| Mullets | ||
| + | | style="border:2px solid lightblue; font-size: 10px; text-align:center"| 291 | ||
| + | | style="border:2px solid lightblue; font-size: 10px; text-align:center"| Giant tiger prawn | ||
| + | | style="border:2px solid lightblue; font-size: 10px; text-align:center"| 717 | ||
| + | | style="border:2px solid lightblue; font-size: 10px; text-align:center"| Scallops | ||
| + | | style="border:2px solid lightblue; font-size: 10px; text-align:center"| 1746 | ||
| + | | style="border:2px solid lightblue; font-size: 10px; text-align:center"| Gracillaria seaweeds | ||
| + | | style="border:2px solid lightblue; font-size: 10px; text-align:center"| 5180 | ||
| + | |- | ||
| + | | style="border:2px solid lightblue; font-size: 10px; text-align:center"| Gilthead seabream | ||
| + | | style="border:2px solid lightblue; font-size: 10px; text-align:center"| 282 | ||
| + | | style="border:2px solid lightblue; font-size: 10px; text-align:center"| Indo-pacific swamp crab | ||
| + | | style="border:2px solid lightblue; font-size: 10px; text-align:center"| 249 | ||
| + | | style="border:2px solid lightblue; font-size: 10px; text-align:center"| Sea mussels | ||
| + | | style="border:2px solid lightblue; font-size: 10px; text-align:center"| 1108 | ||
| + | | style="border:2px solid lightblue; font-size: 10px; text-align:center"| Wakama | ||
| + | | style="border:2px solid lightblue; font-size: 10px; text-align:center"| 2811 | ||
| + | |- | ||
| + | | style="border:2px solid lightblue; font-size: 10px; text-align:center"| Large yellow croaker | ||
| + | | style="border:2px solid lightblue; font-size: 10px; text-align:center"| 254 | ||
| + | | style="border:2px solid lightblue; font-size: 10px; text-align:center"| Green mud crab | ||
| + | | style="border:2px solid lightblue; font-size: 10px; text-align:center"| 159 | ||
| + | | style="border:2px solid lightblue; font-size: 10px; text-align:center"| Constricted tagelus | ||
| + | | style="border:2px solid lightblue; font-size: 10px; text-align:center"| 860 | ||
| + | | style="border:2px solid lightblue; font-size: 10px; text-align:center"| Nori | ||
| + | | style="border:2px solid lightblue; font-size: 10px; text-align:center"| 2220 | ||
| + | |- | ||
| + | | style="border:2px solid lightblue; font-size: 10px; text-align:center"| European seabass | ||
| + | | style="border:2px solid lightblue; font-size: 10px; text-align:center"| 244 | ||
| + | | style="border:2px solid lightblue; font-size: 10px; text-align:center"| | ||
| + | | style="border:2px solid lightblue; font-size: 10px; text-align:center"| | ||
| + | | style="border:2px solid lightblue; font-size: 10px; text-align:center"| Pacific cupped oyster | ||
| + | | style="border:2px solid lightblue; font-size: 10px; text-align:center"| 610 | ||
| + | | style="border:2px solid lightblue; font-size: 10px; text-align:center"| Elkhorn seamoss | ||
| + | | style="border:2px solid lightblue; font-size: 10px; text-align:center"| 1604 | ||
| + | |- | ||
| + | | style="border:2px solid lightblue; font-weight:bold; font-size: 10px; text-align:center"| Total finfish | ||
| + | | style="border:2px solid lightblue; font-weight:bold; font-size: 10px; text-align:center"| 8340 | ||
| + | | style="border:2px solid lightblue; font-weight:bold; font-size: 10px; text-align:center"| Total crustaceans | ||
| + | | style="border:2px solid lightblue; font-weight:bold; font-size: 10px; text-align:center"| 6760 | ||
| + | | style="border:2px solid lightblue; font-weight:bold; font-size: 10px; text-align:center"| Total molluscs | ||
| + | | style="border:2px solid lightblue; font-weight:bold; font-size: 10px; text-align:center"| 17548 | ||
| + | | style="border:2px solid lightblue; font-weight:bold; font-size: 10px; text-align:center"| Total seaweed | ||
| + | | style="border:2px solid lightblue; font-weight:bold; font-size: 10px; text-align:center"| 35013 | ||
| + | |} | ||
| − | + | {| style="border-collapse:collapse; font-size: 12px; background:ivory;" cellpadding=5px align=center width=100% | |
| + | |+ Table 2. Countries with the largest mariculture production in 2020<ref name=FAO>FAO (2022) The State of World Fisheries and Aquaculture 2022. Towards Blue transformation. Rome. https://doi.org/10.4060/cc0461en </ref>. The figures include coastal aquaculture. | ||
| + | |- style="font-weight:bold; font-size: 10px; text-align:center; background:lightblue" | ||
| + | ! width="20% style="border:2px solid blue;"| Finfish | ||
| + | ! width="5% style="border:2px solid blue;"| billion | ||
| + | tonnes | ||
| + | ! width="20% style="border:2px solid blue;"| Crustaceans | ||
| + | ! width="5% style="border:2px solid blue;"| billion | ||
| + | tonnes | ||
| + | ! width="20% style="border:2px solid blue;"| Molluscs | ||
| + | ! width="5% style="border:2px solid blue;"| billion | ||
| + | tonnes | ||
| + | ! width="20% style="border:2px solid blue;"| Seaweed | ||
| + | ! width="5% style="border:2px solid blue;"| billion | ||
| + | tonnes | ||
| + | |- | ||
| + | | style="border:2px solid lightblue; font-size: 10px; text-align:center"| China | ||
| + | | style="border:2px solid lightblue; font-size: 10px; text-align:center"| 1.7 | ||
| + | | style="border:2px solid lightblue; font-size: 10px; text-align:center"| China | ||
| + | | style="border:2px solid lightblue; font-size: 10px; text-align:center"| 1.8 | ||
| + | | style="border:2px solid lightblue; font-size: 10px; text-align:center"| China | ||
| + | | style="border:2px solid lightblue; font-size: 10px; text-align:center"| 1.5 | ||
| + | | style="border:2px solid lightblue; font-size: 10px; text-align:center"| China | ||
| + | | style="border:2px solid lightblue; font-size: 10px; text-align:center"| 20.8 | ||
| + | |- | ||
| + | | style="border:2px solid lightblue; font-size: 10px; text-align:center"| Norway | ||
| + | | style="border:2px solid lightblue; font-size: 10px; text-align:center"| 1.5 | ||
| + | | style="border:2px solid lightblue; font-size: 10px; text-align:center"| Vietnam | ||
| + | | style="border:2px solid lightblue; font-size: 10px; text-align:center"| 1.1 | ||
| + | | style="border:2px solid lightblue; font-size: 10px; text-align:center"| South Korea | ||
| + | | style="border:2px solid lightblue; font-size: 10px; text-align:center"| 0.4 | ||
| + | | style="border:2px solid lightblue; font-size: 10px; text-align:center"| Indonesia | ||
| + | | style="border:2px solid lightblue; font-size: 10px; text-align:center"| 9.6 | ||
| + | |- | ||
| + | | style="border:2px solid lightblue; font-size: 10px; text-align:center"| Chile | ||
| + | | style="border:2px solid lightblue; font-size: 10px; text-align:center"| 1.1 | ||
| + | | style="border:2px solid lightblue; font-size: 10px; text-align:center"| India | ||
| + | | style="border:2px solid lightblue; font-size: 10px; text-align:center"| 0.5 | ||
| + | | style="border:2px solid lightblue; font-size: 10px; text-align:center"| Chile | ||
| + | | style="border:2px solid lightblue; font-size: 10px; text-align:center"| 0.4 | ||
| + | | style="border:2px solid lightblue; font-size: 10px; text-align:center"| South Korea | ||
| + | | style="border:2px solid lightblue; font-size: 10px; text-align:center"| 1.8 | ||
| + | |- | ||
| + | | style="border:2px solid lightblue; font-size: 10px; text-align:center"| Indonesia | ||
| + | | style="border:2px solid lightblue; font-size: 10px; text-align:center"| 0.9 | ||
| + | | style="border:2px solid lightblue; font-size: 10px; text-align:center"| Indonesia | ||
| + | | style="border:2px solid lightblue; font-size: 10px; text-align:center"| 0.9 | ||
| + | | style="border:2px solid lightblue; font-size: 10px; text-align:center"| Japan | ||
| + | | style="border:2px solid lightblue; font-size: 10px; text-align:center"| 0.3 | ||
| + | | style="border:2px solid lightblue; font-size: 10px; text-align:center"| Philippines | ||
| + | | style="border:2px solid lightblue; font-size: 10px; text-align:center"| 1.5 | ||
| + | |} | ||
| Line 110: | Line 239: | ||
====Release of hazardous substances==== | ====Release of hazardous substances==== | ||
| − | [[Image:pollutant.jpg|thumb|left|550px|Table | + | [[Image:pollutant.jpg|thumb|left|550px|Table 3: Chemicals used in mariculture practice that can become pollutants, their sources/uses and impact (CBD, 2004<ref name="CBD"/>).]] |
| − | Harmful algae are often combated with chemicals or other hazardous products. Although many effective aqueous algicidal treatments exist, few are approved for use in open marine systems, due to environmental concerns (Gallardo-Rodrıguez et al. 2019<ref> Gallardo-Rodrıguez JJ, Astuya-Villalon A, Llanos-Rivera A, Avello-Fontalba V, Ulloa-Jofre V (2019) A critical review on control methods for harmful algal blooms. Reviews in Aquaculture 2019, p. 661–684</ref>). However, some antimicrobial chemicals and pesticides are licensed for use in mariculture, specifically for finfish culture. Various synthetic chemicals (see | + | Harmful algae are often combated with chemicals or other hazardous products. Although many effective aqueous algicidal treatments exist, few are approved for use in open marine systems, due to environmental concerns (Gallardo-Rodrıguez et al. 2019<ref> Gallardo-Rodrıguez JJ, Astuya-Villalon A, Llanos-Rivera A, Avello-Fontalba V, Ulloa-Jofre V (2019) A critical review on control methods for harmful algal blooms. Reviews in Aquaculture 2019, p. 661–684</ref>). However, some antimicrobial chemicals and pesticides are licensed for use in mariculture, specifically for finfish culture. Various synthetic chemicals (see Table 3) interfere with HAB cell survival, growth and reproduction. Biochemicals are biodegradable and have, in some cases, lower toxicity to the wider environment. |
Biological control measures further include the application of microbial (viral, bacterial, fungal and/or protistan) parasites that infect HABs and can play a significant role in the natural termination of major blooms (Ross Brown et al., 2020<ref name=RB>Ross Brown A, Lilley M, Shutler J, Lowe C, Artioli Y, Torres R, Berdalet E, Tyler CR (2020). Assessing risks and mitigating impacts of harmful algal blooms on mariculture and marine fisheries. Rev. Aquacult. 12, p. 1663-1688</ref>). | Biological control measures further include the application of microbial (viral, bacterial, fungal and/or protistan) parasites that infect HABs and can play a significant role in the natural termination of major blooms (Ross Brown et al., 2020<ref name=RB>Ross Brown A, Lilley M, Shutler J, Lowe C, Artioli Y, Torres R, Berdalet E, Tyler CR (2020). Assessing risks and mitigating impacts of harmful algal blooms on mariculture and marine fisheries. Rev. Aquacult. 12, p. 1663-1688</ref>). | ||
| Line 160: | Line 289: | ||
*the risk of harmful algal blooms is reduced; | *the risk of harmful algal blooms is reduced; | ||
*access to the lucrative market of eco-friendly foods. | *access to the lucrative market of eco-friendly foods. | ||
| − | This system is considered a good solution by many scientists (Chopin et al., 2001<ref> Chopin T, Buschmann AH, Halling C, Troell M, Kautsky N, Neori A, Kraemer GP, Zertuche-González, Yarish C, Nefus C (2001): Integrating seaweeds into marine aquaculture systems: a key toward sustainability; Journal of Phycology 37, p 975-986</ref>; Neori et al., 2004<ref name="neori"/>; CBD, 2004<ref name="CBD"/>; Troell et al., 2003<ref name="troell03"/>). | + | This system is considered a good solution by many scientists (Chopin et al., 2001<ref> Chopin T, Buschmann AH, Halling C, Troell M, Kautsky N, Neori A, Kraemer GP, Zertuche-González, Yarish C, Nefus C (2001): Integrating seaweeds into marine aquaculture systems: a key toward sustainability; Journal of Phycology 37, p 975-986</ref>; Neori et al., 2004<ref name="neori">Neori A, Chopin T, Troell M, Buschmann AH, Kraemer GP, Halling C, Shpigel M, Yarish C (2004): Integrated aquaculture: rationale, evolution and state of the art emphasizing seaweed biofiltration in modern mariculture; Aquaculture 231, p. 361-391</ref>;; CBD, 2004<ref name="CBD"/>; Troell et al., 2003<ref name="troell03"/>). |
Revision as of 15:34, 20 August 2023
Definition
Mariculture is often defined as aquaculture in marine environments. Some limit mariculture to culture of marine plants and animals in the ocean itself (EEA, 2008[1]). Others also include species from brackish water and include culture methods that take place in salty and brackish water that is situated in the coastal zone (CBD, 2004[2]; Wecker, 2006[3]). Here this wider definition is referred to. Mariculture can be distinguished from capture fisheries by two criteria: ownership of the stock and deliberate intervention in the production cycle (husbandry) (Naylor et al., 2000[4]).
Introduction
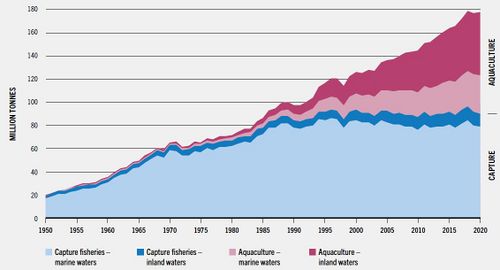
Mariculture includes a wide range of species and culture methods. It is globally a fast-growing activity, see Fig. 1. This is due to the fact that many wild fish stocks are overfished and catches are declining (Wecker, 2006[3]). At the same time the world population is rising and with it the need for dietary protein. The expansion of mariculture can reduce pressure on wild fish, shrimps and molluscs, because they reduce their market price and by this the investments in fishing fleets. However, they can also increase the pressure due to the use of fishmeal in feed for some mariculture-species (Naylor et al., 2000[4]).
Some forms of mariculture provide good quality food and the production is more efficient than that of terrestrial animals; roughly half the level of feed input per unit output is necessary (CBD, 2004[2]). Products obtained from mariculture are not only used for food, but also as raw material for e.g. cosmetics, neutraceuticals, medicines, food additives and many more.
The main species grown in mariculture are listed in Table 1. The countries with the largest production are listed in Table 2. Most mariculture is concentrated in East Asia.
| Finfish | thousand
tonnes |
Crustaceans | thousand
tonnes |
Molluscs | thousand
tonnes |
Seaweed | thousand
tonnes |
|---|---|---|---|---|---|---|---|
| Atlantic salmon | 2720 | Whiteleg shrimp | 5812 | Cupped oyster | 5450 | Japanese kelp | 12470 |
| Milkfish | 1168 | Chinese mitten crab | 776 | Japanese carpet shell | 4266 | Eucheuma seaweeds | 8129 |
| Mullets | 291 | Giant tiger prawn | 717 | Scallops | 1746 | Gracillaria seaweeds | 5180 |
| Gilthead seabream | 282 | Indo-pacific swamp crab | 249 | Sea mussels | 1108 | Wakama | 2811 |
| Large yellow croaker | 254 | Green mud crab | 159 | Constricted tagelus | 860 | Nori | 2220 |
| European seabass | 244 | Pacific cupped oyster | 610 | Elkhorn seamoss | 1604 | ||
| Total finfish | 8340 | Total crustaceans | 6760 | Total molluscs | 17548 | Total seaweed | 35013 |
| Finfish | billion
tonnes |
Crustaceans | billion
tonnes |
Molluscs | billion
tonnes |
Seaweed | billion
tonnes |
|---|---|---|---|---|---|---|---|
| China | 1.7 | China | 1.8 | China | 1.5 | China | 20.8 |
| Norway | 1.5 | Vietnam | 1.1 | South Korea | 0.4 | Indonesia | 9.6 |
| Chile | 1.1 | India | 0.5 | Chile | 0.4 | South Korea | 1.8 |
| Indonesia | 0.9 | Indonesia | 0.9 | Japan | 0.3 | Philippines | 1.5 |
Despite the rapid growth of mariculture and the great potential for food production, only a small part of the world's food supply comes from mariculture. Estimates from around the year 2010 indicate that about 98% of the world's food supply is provided by the terrestrial domain (agriculture). Only 1.4% comes from the marine domain: fishery (~ 1%) and mariculture (~0.4%) (Olsen, 2015[6]). These figures show that the potential of mariculture as a food source is still far underused. The ocean area suitable for mariculture is many times larger than the area currently used (Oyinlola et al., 2018[7]).
Several environmental problems are associated with mariculture. These problems depend on species, culture method, stocking density, feed type, husbandry practice, hydrodynamic site conditions and the sensitivity of the receiving ecosystem (Troell et al., 1999[8]; Wu, 1995[9]). Many of these problems can be mitigated with appropriate measures. Farmers are in general aware that mariculture itself in the long run depends on good quality of the environment.
Mariculture can play an important role, especially in rural areas, for food security, economic and social welfare. In densely populated coastal areas, mariculture is in competition with other human activities for space and other resources. These other activities can for example be: fisheries, tourism, harbour operations, nature conservation and industry. Integrated Coastal Zone Management (ICZM) tries to bring these activities in the coastal zone together in a sustainable way (Wu, 1995[9]; Read and Fernandes, 2003[10]; Wecker, 2006[3]). Legislation on mariculture and its enforcement vary widely in different countries around the world. No further consideration is given here to this topic.
Types of mariculture

Different kinds of mariculture are presented here according to a subdivision by species type. Different types of species require different systems that have different characteristics and effects. Only the most common systems are mentioned (CBD, 2004[2]).
Mollusc Culture
Broodstock/seed supply: Bivalve mollusc larvae are either collected from natural grounds using material to which they adhere or produced in hatcheries by artificial fertilization.
Growout: Larvae that have set to their substrate are grown in hanging cultures (suspended from floating rafts or long lines on strings, trays, stacks or mesh bags), vertical or rack culture (sticks or platforms), bottom culture (shells, stones, rocks or cement slabs added to the ground), or in land-based systems (CBD, 2004[2]).

Crustacean Culture (Fig. 2)
Broodstock/seed supply: In the last century the global industry relied mainly on wild-caught larvae or berried (= egg-carrying) females. Nowadays there is a trend towards hatcheries.
Growout: takes place in earthen ponds, concrete raceways and tanks (CBD, 2004[2]).

Marine Plant Culture (Fig. 3)
This includes seaweed (also called macroalgae, the vast majority of marine plant cultures), microalgae as well as seagrasses.
Broodstock/seed supply: Cultured aquatic plants have complicated life cycles with several intermediate stages. The major source of broodstock is wild collection. Most culture is now dependent on hatchery production of the early life stages (monospores, zoospores, gametophytes, sporophytes) which are attached to growing media and transferred to marine sites. Other propagation methods involve fragmentation.
Growout: Young plants are cultured by 3 different methods: suspended (longline and raft), bottom cultures at the sea (large rocks or artificial shapes of concrete are placed on the seabed) and inland tank cultures (CBD, 2004[2]).
Finfish Culture (Fig. 4)
Broodstock/seed supply: The broodstock can be domesticated or a mix of domesticated and wild animals. Most species are grown from larvae or fry produced in hatcheries. Spawning is often stimulated with a hormone application.
Growout: Cage culture can be divided into inshore and offshore cages and can be fixed, floating or submerged. Inshore cages are located in protected, shallow areas with less water circulation. Offshore cages are located in deep water and open areas with less protection from storm but with better water exchange. Nets and fish pen are located in shallow water and their edges are anchored to the bottom. A typical fish pond system consists of the following basic components: pond compartments enclosed by dikes, canals for supply and drainage of water and gates or water control structures (CBD, 2004[2]).
Enhancement or Sea Ranching is mostly developed with marine finfish. Both terms refer to the deliberate release of organisms from hatcheries into the natural ecosystem. In enhancement, fry are released to restock wild populations. In sea ranching, fish are harvested from artificially enclosed areas (CBD, 2004[2]).
It is also possible to co-culture different species: this will be further described in the section about mitigation.
Fig. 5 illustrates the impacts of different types of mariculture according to the intensity of farming systems.
Environmental risks and impacts
Environmental impacts depend on husbandry parameters (species, culture method, feed type) and the nature of the receiving environment (physical, chemical, biological characteristics). The state of the receiving ecosystem also depends on the release of waste products from other anthropogenic sources (e.g. effluents from industry or human settlements or agricultural runoff).
Nutrient pollution / Eutrophication
Eutrophication defined as nutrient enrichment (mainly N and P) is considered by some the most important pollution threat to marine waters (Wu, 1999[16]; Bouwman et al., 2013[17]). This problem is often mentioned in the context of intensive culture of fish and shrimp, where a lot of artificial feed are used. Waste consists of uneaten feed and faeces moving down into the benthos: below fish cages in areas with low currents waste sedimentation leads to a shift in benthic populations towards pollutant-resistant species. This effect is mostly limited to a distance of 50-100 m from the mariculture facilities. Another part of the waste products consists of CO2, dissolved organic carbon and various soluble nutrients (e.g. ammonia and phosphate) which are dispersed over the water column (CBD, 2004[2]; Troell et al., 1999[8]).
To date, anthropogenic input of nutrients (not only by mariculture) have caused major changes in structure and functioning of phyto- and zooplankton, benthic and fish communities (Wu, 1999[16]; Troell et al., 1999[8]). For example, observations over a two-decade period show that long-term exposure to aquaculture effluents with high nutrient concentrations are a serious threat to coastal ecosystems along the whole Chinese coast, and in particular to seagrass meadows, which have largely disappeared (Thomsen et al., 2020[18]). Areas with limited water exchange are at even greater risk.
Algal blooms can shade seafloor vegetation and when they collapse their decay on the seafloor may lead to hypoxia or anoxia and hence mass mortality of benthos and fish (Troell et al., 2003[19]).
The opposite of eutrophication may occur at intensive open ocean bivalve cultures: They take nutrients away from the marine foodweb. Excessive nutrient depletion limits the growth of other herbivores and phytoplankton and those that live off them. Apart from that, bivalves filter suspended particulate matter and change it into denser particles that fall to the bottom (faecal pellets). This can have an effect on benthic communities as well (CBD, 2004[2]).
Harmful algal blooms (HABs)
The effluents from fish farming have high N/P-ratios, which are considered a likely cause for the development of harmful algal blooms (Fig. 6). Toxins produced by certain species of algae constitute an important public health risk, mainly via human consumption of filter-feeding shellfish contaminated with biotoxins (Wu, 1995[9]). Precautionary closure of mariculture farms upon the detection of HABs are a major risk and cost factor for the mariculture industry, estimated at several billion US$ per year worldwide (Berdalet et al., 2016[20]).
Release of hazardous substances

Harmful algae are often combated with chemicals or other hazardous products. Although many effective aqueous algicidal treatments exist, few are approved for use in open marine systems, due to environmental concerns (Gallardo-Rodrıguez et al. 2019[21]). However, some antimicrobial chemicals and pesticides are licensed for use in mariculture, specifically for finfish culture. Various synthetic chemicals (see Table 3) interfere with HAB cell survival, growth and reproduction. Biochemicals are biodegradable and have, in some cases, lower toxicity to the wider environment.
Biological control measures further include the application of microbial (viral, bacterial, fungal and/or protistan) parasites that infect HABs and can play a significant role in the natural termination of major blooms (Ross Brown et al., 2020[22]).
Spreading of parasites and diseases
Due to crowded and stressful conditions in intensive mariculture there are frequent outbreaks of diseases. The pathogens can be dispersed to previously disease-free regions by transport of hatchery products like shrimp-postlarvae. When animals with infections or parasites escape, the pathogens can be spread to wild stocks (CBD, 2004[2]).
Escapes / Aliens / Biodiversity / Genetics
Non-native species resulting from escaped culture stocks can establish far from their home range. In some cases this may enrich biodiversity, but often they predate on or compete with native species and could eventually eliminate these (CBD, 2004[2]). For example, surveys show the dispersal of alien invasive species from shellfish culture areas rafting on floating litter in the Venetian lagoon and the Portuguese Algarve region, including the notorious nuisance species H. sanctaecrucis (Rech et al., 2018[23]). It is estimated that escapes of non-native species from finfish farms threaten almost one third of the ocean ecosystems (Atalah and Sanchez-Perez, 2020[24]). See also the article Non-native species invasions.
There is also concern that the escaped fish might lead to a decrease in intraspecific genetic variability via mixing of escaped cultured animals with wild stocks. Adaptive features of local fish populations can be lost by interbreeding with genetically less diverse and less adapted farmed fish (Miralles et al., 2016[25]). Fishery research in the sea around the Faroe islands showed that 20-30% of salmon there are escapees from farms (Read and Fernandes, 2003[10]). Genetically modified fish can also become a problem in the future (CBD, 2004[2]).
Farming up and fishing down the food chain / Food security
High-value marine carnivorous finfish need animal sources of protein. Most of this comes from marine fish in the form of fish meal. The fish meal is made from small pelagic wild fish e.g. anchoveta and atlantic herring. This practice raises two main issues. One is that less food is left for marine predators like seals and seabirds and for commercially valuable predatory fish like cod (CBD, 2004[2]). The other concern is human food security. Often 2-5 times more fish protein is put into the farmed species than is supplied by the farmed product. Such a concern does not exist for herbivorous filter feeders, who are net protein producers (Naylor et al., 2000[4]). Culture of more low trophic level species or groups (e.g., omnivore fish, mollusks and seaweed) should be stimulated. Unfortunately, there are few attractive herbivorous fish species in the marine environment. See also Overexploitation.
Catching broodstock from the wild
This practice entails several threats to the environment. The natural stocks of the target specimen are depleted, leading to problems for species that normally feed on them (e.g. shrimp larvae are a food source for many organisms). There are also other side effects: bycatch may be very high in some cases and sometimes destructive gear like dredge nets is used (CBD, 2004[2]).
Habitat degradation / modification
Depending on the cultivation method, mariculture can take a lot of space, which can perturb migratory routes, feeding patterns and reproduction of non-target species. One example is the conversion of mangroves into shrimp ponds. Once in operation, the effluents of these ponds pose a threat to adjacent mangrove ecosystems. Saltwater intrusion due to active pumping of groundwater into the ponds can cause additional problems (Páez-Osuna, 2001[26]; CBD, 2004[2]).
Acoustic disturbance
Underwater exploders are sometimes used in mariculture to deter predators from the farmed animals. This can also stress non-target animals (CBD, 2004[2]).
Possibilities for mitigation
Many of the aforementioned possible negative environmental impacts of mariculture can be mitigated. In the following section some possible measures are discussed.
Integrated multi-trophic aquaculture (IMTA)
Integrated multi-trophic aquaculture (IMTA) is a form of mariculture that imitates natural ecosystems. IMTA employs cultureable ‘extractive’ species (e.g. suspended bivalve shellfish and macroalgae, and benthic deposit feeders) to remove/reuse waste nutrient material discarded from the culturing of ‘fed’ species (finfish and crustaceans) thereby providing a self-sustaining and more productive food web. China has some of the world’s largest and longest established IMTA systems (Wartenberg et al. 2017[27]).
The effluents from intensively fed cultures of finfish or shrimps are taken up by bivalves and plants. Marine plants use sunlight and assimilate dissolved inorganic nutrients from the water, while bivalves filter organic suspended particles which can be left-over feed or phytoplankton from the effluents. The marine plants can be phytoplankton that is then eaten by the bivalves or seaweeds that can be sold (like the bivalves). Integrated aquaculture includes the production of larvae in mariculture facilities instead of taking them from the wild (CBD, 2004[2]).
Integrated aquaculture has many advantages:
- waste of one species can be converted into products that have an economic value, providing a higher income and diversification of the mariculture production while also reducing financial risks;
- the adverse environmental impacts of intensive culture of carnivores are reduced and sustainability can be reached, as seaweeds not only absorb the nutrient release from the fish and shrimp cultures, but also reduce other impacts related to dissolved oxygen, acidity and CO2;
- the risk of harmful algal blooms is reduced;
- access to the lucrative market of eco-friendly foods.
This system is considered a good solution by many scientists (Chopin et al., 2001[28]; Neori et al., 2004[29];; CBD, 2004[2]; Troell et al., 2003[19]).
Use of enclosed recirculating systems for shrimp and finfish
Enclosed systems prevent escapes and aerated settling tanks or other (bio-)filters prevent most particulate nutrients and parts of the dissolved nutrients to enter the natural ecosystems. A problem is that they require high initial investments (CBD, 2004[2]).
Site selection
Reduced hydrodynamic flows are known to lead to reduced turbulence, which in turn tends to promote the blooming of dinoflagellate species, including HAB species (see Harmful algal bloom). Mariculture structures, including longlines for shellfish and kelp and net pens for finfish can significantly change surface current speed and direction, induce down-welling, increase stratification and reduce water exchange in sheltered and enclosed bays. An example is to choose sites with high water exchange rates and currents that dilute the waste (CBD, 2004[2]). However, whether dilution is a long-term-solution, is questionable.
Use of hydraulic devices
The development of harmful algal blooms can also be counteracted by water column mixing using water or air pumping systems. This leads to disruption of thermal stratification and impairment of algal buoyancy or alteration of their daily migration patterns, removing them from the photic zone and preventing photosynthesis.
Removal of harmful algae
Direct removal of harmful algae from the water column can be achieved by hydrodynamic separation, centrifugation, pump filtration, plankton net trawling or membrane filtration. Physical control methods can remove HABs efficiently and are used operationally as a last resort in mariculture, but they can be costly, lack specificity to HABs, and are generally less effective in coastal situations in comparison with enclosed or semi-enclosed aquatic systems.
The use of clay to induce bloom flocculation has been shown to be an effective measure for HAB control in the open sea. Subsequent sinking and burial of HAB cells and/or cysts can be followed by dredging and physical or chemical treatment before discharging the sediments back to the removal site (Ross Brown et al., 2020[22]). Modified clays can kill HAB cells, adsorb and remove extracellular HAB toxins and particulate nutrients, and also reduce HAB toxin accumulation in benthic filter-feeding bivalves. They are employed as a standard method for controlling HABs in China, since 2014 (Yu et al. 2017[30]).
Reduction of eutrophication impacts
Measures consist of carefully selecting farmed species and setting a limit to stocking density. The carrying capacity of the ecosystem to process waste products should also be taken into account (CBD, 2004[2]). The effectiveness of these measures is difficult to assess, however, especially in coastal zones with cumulative pressure from other anthropogenic activities.
Another mitigation technique consists of fixing cages at only one mooring on a long line so that they can float over a large area (moved by e.g. wind and tidal currents). This may help to reduce local amounts of sedimentation and prevent degradation of benthic habitats (Goudey et al., 2001[31]).
Feeding management: to reduce waste ...
One example is the improvement of feed composition by reducing N and P in the diets (N is often the limiting nutrient for phytoplankton growth in the open sea). Another example is the use of efficient strains of the farmed species. In shrimp ponds, natural feed items like zooplankton and benthic organisms can be used as a supplement to artificial diets (CBD, 2004[2]). It is important to better inform farm workers and to raise their awareness on these issues.
... and improve food security:
Reducing fish meal in feed and improving feed efficiency are already priorities in the mariculture industry as feed is the largest cost item in many intensive culture systems and the prices of fish meal continue to rise (Naylor et al., 2000[4]). Farming fish of low trophic levels and the reduction of fish meal and oils inputs in feed should be stimulated (Naylor et al., 2000[4]).
Reducing disease outbreaks and transmission and the use of pesticides, piscicides and parasiticides and antibiotics
This can be achieved by establishing lower stocking densities and keeping larger distances between individual farms. Probiotics can be used to improve the water quality. Emerging guidelines for assuring minimal impacts from offshore mariculture on water quality and pelagic and benthic communities recommend minimum water depths (twice the depth of mariculture infrastructure) and minimum water flow rates (>0.05 m/s). In such localities, the probability of ecological effects on neighbouring natural habitats diminishes significantly beyond a distance of 90 m (Froehlich et al., 2017[32]). Vaccination is available against some important infectious diseases (Hoelzer et al., 2018[33]).
Reducing the use of hormones
Alternatives can be proper genetic selection programmes and the use of photoperiod management in the industrial production of salmon (CBD, 2004[2]).
Monitoring of harmful algal blooms
Monitoring and predictive modelling can greatly contribute to controlling risks and impacts of HABs. In situ monitoring for HAB species abundance and phycotoxin concentrations in (shell)fish is the principal method for ‘official control’ monitoring and safeguarding of food fish safety for human consumption in Europe, North America, Asia and Australasia[22]. In situ monitoring is generally conducted via the collection and analysis of representative field samples, often by means of autonomous in situ molecular (qPCR) and flow cytometry methods, supplemented with microscopic analysis for phytoplankton identification and enumeration, and mass spectrometric analysis for phycotoxin identification and quantitation. In Europe, routine HAB monitoring (EU Directives 2000/60/EC and 2006/113/EC) is used to quantify HAB species abundance and phytotoxin levels. Regulations force closure of mariculture farms when certain levels of biotoxins are exceeded (O'Mahony, 2018[34]).
Under certain circumstances harmful algal blooms can also be detected by remote sensing techniques. Images of ocean colour from visible and infrared spectrum wavelengths can be correlated statistically with HABs events or in some cases the HAB species can be observed if they are spectrally distinct (https://www.shelleye.org/index; https://www.s3euro hab.eu/en/) (Ross Brown et al., 2018[22]). For some species (for example Karenia mikimotoi and K. brevis) correlations have been found between ocean colour, chlorophyll and algal biomass (El-Habashi et al., 2017[35]). In general, HAB species that are detectable by remote sensing are those that form significant blooms of >1000 cells/mL at the sea surface or near-surface. Satellite imaging however cannot detect species that form harmful blooms at greater depths or at low densities.
Observations can be supplemented with models to forecast HAB development and evolution. These numerical models are generally better at predicting HAB initiation than HAB termination, but in any event forecasting is generally limited to 1 week in advance (Schmidt et al. 2018[36]), which corresponds with general extent and accuracy of meteorological forecasting.
See also
Internal Links
- Effects of fisheries on marine biodiversity
- Harmful algal bloom
- ALGADEC - Detection of toxic algae with a semi-automated nucleic acid biosensor
- Non-native species invasions
- Seaweed (macro-algae) ecosystem services
External links
- The State of World Fisheries and Aquaculture, FAO 2014
- Assessment of Impacts of Mariculture, OSPAR Commission 2009
References
- ↑ European Environmental agency; https://www.eea.europa.eu/help/glossary/eea-glossary/mariculture
- ↑ 2.00 2.01 2.02 2.03 2.04 2.05 2.06 2.07 2.08 2.09 2.10 2.11 2.12 2.13 2.14 2.15 2.16 2.17 2.18 2.19 2.20 2.21 2.22 2.23 2.24 Secretariat of the Convention on Biological Diversity (2004): Solutions for sustainable mariculture-avoiding the adverse effects of mariculture on biological diversity, CBD Technical Series No. 12
- ↑ 3.0 3.1 3.2 Wecker B (2006): Nährstofffluss in einer geschlossenen Kreislaufanlage mit integrierter Prozesswasserklärung über Algenfilter-Modell und Wirklichkeit.; https://macau.uni-kiel.de/receive/dissertation_diss_00001878
- ↑ 4.0 4.1 4.2 4.3 4.4 Naylor RL, Goldburg RJ, Primavera JH, Kautsky N, Beveridge MCM, Clay J, Folke C, Lubchenko J, Mooney H, Troell M (2000): Effect of aquaculture on world fish supplies; Nature 405, p. 1017-1024
- ↑ 5.0 5.1 5.2 FAO (2022) The State of World Fisheries and Aquaculture 2022. Towards Blue transformation. Rome. https://doi.org/10.4060/cc0461en
- ↑ Olsen Y (2015) How can mariculture better help feed humanity? Front. Mar.Science 2: 46. doi: 10.3389/fmars.2015.00046
- ↑ Oyinlola MA, Reygondeau G, Wabnitz CCC, Troell M, Cheung WWL (2018) Global estimation of areas with suitable environmental conditions for mariculture species. PLoS ONE 13(1): e0191086. https://doi.org/10.1371/journal.pone.0191086
- ↑ 8.0 8.1 8.2 Troell M, Rönnbäck P, Halling C, Kautsky N, Buschmann A (1999): Ecological engineering in aquaculture: use of seaweeds for removing nutrients from intensive aquaculture; Journal of Applied Phycology 11, p. 89-97
- ↑ 9.0 9.1 9.2 Wu RSS (1995): The environmental impact of marine fish culture: Towards a sustainable future; Marine Pollution Bulletin 31, p. 159-166
- ↑ 10.0 10.1 Read P, Fernandes T (2003): Management of environmental impacts of marine aquaculture in Europe; Aquaculture 226, p. 139-163
- ↑ http://www.fishfarming.com/shrimp.html, 01/28/08
- ↑ http://www.seaweed.ie/aquaculture/LowvsHigh.php
- ↑ http://www.dfo-mpo.gc.ca/index-eng.htm
- ↑ Tacon AGJ, Forster IP (2003): Aquafeeds and the environment: political implications; Aquaculture 226, p. 181-189
- ↑ http://serc.carleton.edu SERC Carleton
- ↑ 16.0 16.1 Wu RSS (1999): Eutrophication, Water Borne Pathogens and Xenobiotic Compounds: Environmental Risks and Challenges; Marine Pollution Bulletin 39, p. 11-22
- ↑ Bouwman L, Beusen A, Glibert PM, Overbeek C, Pawlowski M, Herrera J, Mulsow S, Yu R and Zhou M (2013). Mariculture: significant and expanding cause of coastal nutrient enrichment. Environmental Research Letters 8:0044026
- ↑ Thomsen E, Herbeck LS and Jennerjahn TC (2020) The end of resilience: Surpassed nitrogen thresholds in coastal waters led to severe seagrass loss after decades of exposure to aquaculture effluents. Marine Environmental Research 160, 104986
- ↑ 19.0 19.1 Troell M, Halling C, Neori A, Chopin T, Buschmann AH, Kautsky N, Yariah C (2003): Integrated mariculture: asking the right questions; Aquaculture 226, p. 69-90
- ↑ Berdalet, E., Fleming, L.E., Gowen, R., Davidson, K., Hess, P., Backer, L.C., Moore, S.K., Hoagland, P. and Enevoldsen, H. 2016. Marine harmful algal blooms, human health and wellbeing: challenges and opportunities in the 21st century. J. Mar. Biol. Assoc. U.K. 96. p. 61–91
- ↑ Gallardo-Rodrıguez JJ, Astuya-Villalon A, Llanos-Rivera A, Avello-Fontalba V, Ulloa-Jofre V (2019) A critical review on control methods for harmful algal blooms. Reviews in Aquaculture 2019, p. 661–684
- ↑ 22.0 22.1 22.2 22.3 Ross Brown A, Lilley M, Shutler J, Lowe C, Artioli Y, Torres R, Berdalet E, Tyler CR (2020). Assessing risks and mitigating impacts of harmful algal blooms on mariculture and marine fisheries. Rev. Aquacult. 12, p. 1663-1688
- ↑ Rech S, Salmina S, Borrell Pichs, YJ and Garcia-Vazquez E (2018) Dispersal of alien invasive species on anthropogenic litter from European mariculture areas. Marine Pollution Bulletin 131, p. 10–16
- ↑ Atalah J and Sanchez-Perez P (2020) Global assessment of ecological risks associated with farmed fish escapes. Global Ecology and Conservation 21, e00842
- ↑ Miralles, L, Mrugala A, Sanchez-Jerez , Juanes F. and Garcia-Vazquez E. (2016) Potential impact of Mediterranean aquaculture on the wild predatory bluefish. Mar. Coast. Fish. 8, p. 92-99
- ↑ Páez-Osuna F (2001): The environmental impact of shrimp aquaculture: a global perspective; Environmental Pollution 112, p. 229-231
- ↑ Wartenberg R, Feng L, Jun WuJ, Mak YL, Chan LL, Telfer TC et al. (2017) The impacts of suspended mariculture on coastal zones in China and the scope for Integrated Multi-Trophic Aquaculture. Ecosystem Health and Sustainability 3: 1340268
- ↑ Chopin T, Buschmann AH, Halling C, Troell M, Kautsky N, Neori A, Kraemer GP, Zertuche-González, Yarish C, Nefus C (2001): Integrating seaweeds into marine aquaculture systems: a key toward sustainability; Journal of Phycology 37, p 975-986
- ↑ Neori A, Chopin T, Troell M, Buschmann AH, Kraemer GP, Halling C, Shpigel M, Yarish C (2004): Integrated aquaculture: rationale, evolution and state of the art emphasizing seaweed biofiltration in modern mariculture; Aquaculture 231, p. 361-391
- ↑ Yu Z, Song X, Cao X, Liu Y (2017) Mitigation of harmful algal blooms using modified clays: theory, mechanisms, and applications. Harmful Algae 69, p. 48–64
- ↑ Goudey CA, Loverich G, Kite-Powell H, Costa-Pierce BA (2001): Mitigating the environmental effects of mariculture through single-point moorings (SPMs) and drifting cages; ICES Journal of Marine Science 58, p. 497-503
- ↑ Froehlich HE, Smith A, Gentry RR, Halpern BS (2017) Offshore Aquaculture: I Know It When I See It. Frontiers in Marine Science 4, 154
- ↑ Hoelzer K, Bielke L, Blake, DP, Cox E, Cutting SM, Devriendt B, Erlacher-Vindel E, Goossens E, Karaca K, Lemiere S, Metzner M, Raicek M, Collell Surinach M, Wong NM, Gay C and Van Immerseel F (2018): Vaccines as alternatives to antibiotics for food producing animals. Part 1: challenges and needs. Vet. Res. 2018, p. 49-64 https://doi.org/10.1186/s13567-018-0560-8
- ↑ O'Mahony M. (2018) EU Regulatory Risk Management of Marine Biotoxins in the Marine Bivalve Mollusc Food-Chain. Toxins, 10(3), 118
- ↑ El-Habashi A, Duran CM, Lovko V, Tomlinson MC, Stumpf RP, Ahmed S (2017) Satellite retrievals of Karenia brevis harmful algal blooms in the West Florida shelf using neural networks and impacts of temporal variabilities. Journal of Applied Remote Sensing 11, 032408
- ↑ Schmidt W, Evers-King HL, Campos CJA, Jones DB, Miller PI, Davidson K et al. (2018) A generic approach for the development of short-term predictions of E. coli and biotoxins in shellfish; application to a coastal bay and an estuary. Aquaculture and Environment Interactions 10, p. 173–185
Please note that others may also have edited the contents of this article.
|
