Difference between revisions of "Isoproturon"
(→Notes) |
(lgae and) |
||
| Line 16: | Line 16: | ||
Isoproturon mainly enters the environment during its application as an agricultural herbicide, but releases may also occur during manufacture, transportation and storage<ref name = ea>[http://www.environment-agency.gov.uk/business/topics/pollution/39121.aspx www.environment-agency.gov.uk August 18 2009]</ref>. | Isoproturon mainly enters the environment during its application as an agricultural herbicide, but releases may also occur during manufacture, transportation and storage<ref name = ea>[http://www.environment-agency.gov.uk/business/topics/pollution/39121.aspx www.environment-agency.gov.uk August 18 2009]</ref>. | ||
| − | Isoproturon has a low tendency to [[adsorption|adsorb]] to soils and is therefore quite able to enter in water bodies | + | Isoproturon has a low tendency to [[adsorption|adsorb]] to soils and is therefore quite able to enter in water bodies despite its has a rather low water solubility (70.2 mg/l). Its [[half-life]] in water is 30 days, in soils 40 days<ref name = back>[http://www.who.int/water_sanitation_health/dwq/chemicals/isoproturon.pdf WHO 2003 Background document for development of WHO Guidelines for Drinking-water Quality]</ref>. |
| − | Due to its low affinity for organic matter it is not expected to have a high tendency towards [[bioaccumulation]] or [[biomagnification]]. Significant bioaccumulation might however occur in certain species. <ref>[http://cat.inist.fr/?aModele=afficheN&cpsidt=13698291 MERLIN Gerard, VUILLOD Maryline, LISSOLO Thierry, CLEMENT Bernard 2002 Fate and bioaccumulation of isoproturon in outdoor aquatic microcosms; Environmental toxicology and chemistry] </ref> | + | Due to its low affinity for organic matter it is not expected to have a high tendency towards [[bioaccumulation]] or [[biomagnification]]. Significant bioaccumulation might however occur in certain [[species]]. <ref>[http://cat.inist.fr/?aModele=afficheN&cpsidt=13698291 MERLIN Gerard, VUILLOD Maryline, LISSOLO Thierry, CLEMENT Bernard 2002 Fate and bioaccumulation of isoproturon in outdoor aquatic microcosms; Environmental toxicology and chemistry] </ref> |
| − | Isoproturon is shown to be very toxic for oysters which | + | Isoproturon is shown to be very toxic for algae and oysters which experience acute toxicity at concentrations above 13 µg/l and 370 µg/l respectively. Long term exposure to concentrations above 1 mg/l might affect the growth of fishes, although they only experience acute toxicity at concentrations above 18 mg/l<ref name="EU">[http://ec.europa.eu/food/plant/protection/evaluation/existactive/list1-41_en.pdf Review report for the active substance isoproturon, adopted December 7 2001]</ref>. |
| − | <ref name="EU">[http://ec.europa.eu/food/plant/protection/evaluation/existactive/list1-41_en.pdf Review report for the active substance isoproturon, adopted December 7 2001]</ref> | ||
| − | In fresh water concentrations of 0,125 µg/l have been recorded.<ref name = back>[http://www.who.int/water_sanitation_health/dwq/chemicals/isoproturon.pdf WHO 2003 Background document for development of WHO Guidelines for Drinking-water Quality]</ref> | + | In fresh water, concentrations of 0,125 µg/l have been recorded.<ref name = back>[http://www.who.int/water_sanitation_health/dwq/chemicals/isoproturon.pdf WHO 2003 Background document for development of WHO Guidelines for Drinking-water Quality]</ref> |
<P> | <P> | ||
Revision as of 07:32, 31 August 2009
Definition of isoproturon:
Isoproturon is a selective, systemic herbicide used in the control of annual grasses and broad-leaved weeds in cereals[1]. Pure isoproturon occurs as colourless crystals which melt at 158°C. It's slightly soluble in water and polar organic solvents[2].
This is the common definition for isoproturon, other definitions can be discussed in the article
|
Notes
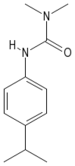
| Isoproturon |
|---|
|
| Formula |
| C12H18N2O |
Isoproturon mainly enters the environment during its application as an agricultural herbicide, but releases may also occur during manufacture, transportation and storage[2]. Isoproturon has a low tendency to adsorb to soils and is therefore quite able to enter in water bodies despite its has a rather low water solubility (70.2 mg/l). Its half-life in water is 30 days, in soils 40 days[3].
Due to its low affinity for organic matter it is not expected to have a high tendency towards bioaccumulation or biomagnification. Significant bioaccumulation might however occur in certain species. [4]
Isoproturon is shown to be very toxic for algae and oysters which experience acute toxicity at concentrations above 13 µg/l and 370 µg/l respectively. Long term exposure to concentrations above 1 mg/l might affect the growth of fishes, although they only experience acute toxicity at concentrations above 18 mg/l[5].
In fresh water, concentrations of 0,125 µg/l have been recorded.[3]
Environmental standards and legislation
Included in the water framework list of priority substances
References
- ↑ WHO 2003 chemical fact sheet
- ↑ 2.0 2.1 www.environment-agency.gov.uk August 18 2009
- ↑ 3.0 3.1 WHO 2003 Background document for development of WHO Guidelines for Drinking-water Quality
- ↑ MERLIN Gerard, VUILLOD Maryline, LISSOLO Thierry, CLEMENT Bernard 2002 Fate and bioaccumulation of isoproturon in outdoor aquatic microcosms; Environmental toxicology and chemistry
- ↑ Review report for the active substance isoproturon, adopted December 7 2001