Difference between revisions of "Free cyanide"
(ref +ref) |
|||
| Line 1: | Line 1: | ||
| − | Cyanide (CN<sup>-</sup>) most commonly occurs as hydrogen cyanide (HCN) and its salts: sodium cyanide (NaCN) and potassium cyanide (KCN). | + | === === |
| + | {{Definition|title= free cyanide | ||
| + | |||
| + | |definition=Cyanide (CN<sup>-</sup>) most commonly occurs as hydrogen cyanide (HCN) and its salts: sodium cyanide (NaCN) and potassium cyanide (KCN). The term free cyanide refers to the cyanide ion and hydrogen cyanide. Cyanides are widely spread in nature, arising from both natural and [[anthropogenic]] sources. Cyanides are produced by certain bacteria, fungi, and algae. Hydrogen cyanide is a colourless liquid/gas with a characteristic odour of bitter almonds.<ref name="ATSDR">[http://www.atsdr.cdc.gov/toxprofiles/tp8.pdf ATSDR 2006 TOXICOLOGICAL PROFILE FOR CYANIDE]</ref> }} | ||
| + | |||
| + | ==Notes== | ||
| + | |||
| + | {| class="toccolours" border="1" style="float: right; clear: right; margin: 0 0 1em 1em; border-collapse: collapse;" | ||
| + | ! bgcolor="#FF8888" | Hydrogen cyanide | ||
| + | |- | ||
| + | | align="center" bgcolor="#FFFFFF" | [[Image:cyanide.png|200px|cyanide ]] | ||
| + | |- | ||
| + | ! bgcolor="#8888FF" | Formula | ||
| + | |- | ||
| + | | align="center" | HCN | ||
| + | |- | ||
| + | |} | ||
| + | |||
| + | Metal finishing and organic chemical industries as well as iron and steel production are major sources of cyanide releases to the aquatic environment, while most of atmospheric cyanide comes from car emissions. In air, cyanide is present mainly as hydrogen cyanide, mainly in gas form, which is very stable (it takes between 1 and 3 years to half its concentration), but a small part forms dust particles which can settle over land or water. In water cyanide doesn't dissolve, but can mix. It weakly [[adsorption|adsorbs]] to particulate matter or soils. In surface water most cyanide will form hydrogen cyanide and evaporate rapidly (concentrations halved in less than 4 days), some can also be biodegraded. <ref name="ATSDR">[http://www.atsdr.cdc.gov/toxprofiles/tp8.pdf ATSDR 2006 TOXICOLOGICAL PROFILE FOR CYANIDE]</ref> | ||
| + | |||
| + | Cyanide doesn't have a tendency to [[bioaccumulation|bioaccumulate]].<ref name="ATSDR">[http://www.atsdr.cdc.gov/toxprofiles/tp8.pdf ATSDR 2006 TOXICOLOGICAL PROFILE FOR CYANIDE]</ref> | ||
| + | |||
| + | Concentrations above 100µg/l can be toxic for zooplankton, fishes only die when exposed to concentrations above 150 µg/l. Molluscs tollerate concentrations up to 300 µg/l. <ref name="pest">[http://www.pesticideinfo.org/Detail_Chemical.jsp?Rec_Id=PC41235 www.pesticideinfo.org August 24 2009]</ref> | ||
| + | |||
| + | |||
| + | The mean concentration of cyanide in surface water is 3,5 µg/l. Concentrations in waste waters can reach up to 120 mg/l.<ref name="ATSDR">[http://www.atsdr.cdc.gov/toxprofiles/tp8.pdf ATSDR 2006 TOXICOLOGICAL PROFILE FOR CYANIDE]</ref> | ||
| + | <P> | ||
| + | <BR> | ||
| + | <P> | ||
| + | == Environmental standards and legislation == | ||
| + | |||
| + | [[List of priority substances|Included in the water framework list of priority substances]] | ||
| + | <P> | ||
| + | <BR> | ||
| + | <P> | ||
| + | == See also == | ||
| + | |||
| + | [http://www.vliz.be/projects/endis/EDnorth.php?showchemprop=true&showeffects=true&chemeffects=true&chemid=211 Cyanide on ED North Database] | ||
| + | <P> | ||
| + | <BR> | ||
| + | <P> | ||
| + | ==References== | ||
| + | <references/> | ||
Revision as of 14:41, 24 August 2009
Definition of free cyanide:
Cyanide (CN-) most commonly occurs as hydrogen cyanide (HCN) and its salts: sodium cyanide (NaCN) and potassium cyanide (KCN). The term free cyanide refers to the cyanide ion and hydrogen cyanide. Cyanides are widely spread in nature, arising from both natural and anthropogenic sources. Cyanides are produced by certain bacteria, fungi, and algae. Hydrogen cyanide is a colourless liquid/gas with a characteristic odour of bitter almonds.[1]
This is the common definition for free cyanide, other definitions can be discussed in the article
|
Notes
| Hydrogen cyanide |
|---|
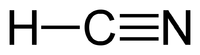
|
| Formula |
| HCN |
Metal finishing and organic chemical industries as well as iron and steel production are major sources of cyanide releases to the aquatic environment, while most of atmospheric cyanide comes from car emissions. In air, cyanide is present mainly as hydrogen cyanide, mainly in gas form, which is very stable (it takes between 1 and 3 years to half its concentration), but a small part forms dust particles which can settle over land or water. In water cyanide doesn't dissolve, but can mix. It weakly adsorbs to particulate matter or soils. In surface water most cyanide will form hydrogen cyanide and evaporate rapidly (concentrations halved in less than 4 days), some can also be biodegraded. [1]
Cyanide doesn't have a tendency to bioaccumulate.[1]
Concentrations above 100µg/l can be toxic for zooplankton, fishes only die when exposed to concentrations above 150 µg/l. Molluscs tollerate concentrations up to 300 µg/l. [2]
The mean concentration of cyanide in surface water is 3,5 µg/l. Concentrations in waste waters can reach up to 120 mg/l.[1]
Environmental standards and legislation
Included in the water framework list of priority substances
See also