Difference between revisions of "Dichloromethane"
| Line 8: | Line 8: | ||
! bgcolor="#FF8888" | Dichloromethane | ! bgcolor="#FF8888" | Dichloromethane | ||
|- | |- | ||
| − | | align="center" bgcolor="#FFFFFF" | [[Image:Dichloromethane. | + | | align="center" bgcolor="#FFFFFF" | [[Image:Dichloromethane.PNG|150px|Dichloromethane ]] |
|- | |- | ||
! bgcolor="#8888FF" | Formula | ! bgcolor="#8888FF" | Formula | ||
Revision as of 10:40, 14 August 2009
Definition of dichloromethane:
Dichloromethane , also known as methylene chloride, is a colourless liquid that has a mild sweet odour, evaporates easily, and does not burn easily. It is widely used as an industrial solvent and as a paint stripper. It can be found in certain aerosol and pesticide products and is used in the manufacture of photographic film. The chemical may be found in some spray paints, automotive cleaners, and other household products. Methylene chloride does not appear to occur naturally in the environment. It is made from methane gas or wood alcohol. [1]
This is the common definition for dichloromethane, other definitions can be discussed in the article
|
Notes
| Dichloromethane |
|---|
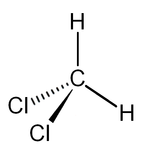
|
| Formula |
| CH2Cl2 |
American production grow steadily during the 1970s and early 1980s to reach a peak production of 281.000 tonnes in 1984. Due to a decrease in demand production dropped to 181.000 tonnes in 1994. European production decreased from an estimated 200.000 tonnes in 1984 to 138.000 tonnes in 1996. Environmental emissions in Europe during the 1990s were estimated to be around 44,6 tonnes a year. Most of the methylene chloride released to the environment results from its use as an end product by various industries and the domestic use of aerosol products and paint removers. Due to its high volatility it is mainly released to the air, and to a lesser extent to water and soil. In the atmosphere it is degraded by sunlight and by reactions with other chemicals. It takes between 53 and 127 days to half its atmospheric concentration. It has a moderate water solubility of 20 g/l and a low tendency to adsorb to particles and sediments. Because of its high volatility dichloromethane is expected to evaporate rapidly from water bodies. It is also degraded in water between 1 and 6 days by reactions with other chemicals or biodegradation by bacteria.
It has a very low tendency to bioaccumulate and is therefore not expected to biomagnify through food chains.[1]
Marine fish seem to start dying at concentrations above 97 mg/l, marine invertebrates at concentrations above 109 mg/l. Marine algae appear most tolerant as they can survive concentrations up to 662 mg/l.[2]
It is suspected that dichloromethane in the North Sea might reach concentrations up to 1 µg/l in heavily polluted coastal areas, although concentrations in polluted estuaries typically range around 0,1 µg/l.[2]
Environmental standards and legislation
Included in the water framework list of priority substances