Difference between revisions of "Diuron"
(→Notes) |
(→Notes) |
||
| Line 21: | Line 21: | ||
In water durion has a low tendency to [[adsorption|adsorb]] to particles and organic matter, dispite its relatively low water solubility of 42 mg/l. It's a stable molecule in terrestrial systems but can be biodegraded by micro-organisms. It is expected to be much less stable in aquatic systems, with a [[half-life]] of 30 days (according to lab experiments). | In water durion has a low tendency to [[adsorption|adsorb]] to particles and organic matter, dispite its relatively low water solubility of 42 mg/l. It's a stable molecule in terrestrial systems but can be biodegraded by micro-organisms. It is expected to be much less stable in aquatic systems, with a [[half-life]] of 30 days (according to lab experiments). | ||
| − | Although it has a low potential towards [[bioaccumulation]], it probably doesn't bioaccumulate or [[biomagnification|biomagnify]] in wild aquatic populations | + | Although it has a low potential towards [[bioaccumulation]], it probably doesn't bioaccumulate or [[biomagnification|biomagnify]] in wild aquatic populations<ref>[http://www.wsdot.wa.gov/maintenance/pdf/diuron.pdf Washington State Department of Transportation February 2006 Diuron Roadside Vegetation Management Herbicide Fact Sheet]</ref>. |
| − | It has a low toxicity for [[pollution and marine mammals|mammals]], as doses above 3,4 g per kg body weight are needed to induce acute toxicity. Some [[pollution and pelagic fishes|fish]] and marine invertebrate [[species ]] experience acute toxic effects at diuron concentrations above 4,3 mg/l and 1 mg/l respectively | + | It has a low [[toxic|toxicity]] for [[pollution and marine mammals|mammals]], as doses above 3,4 g per kg body weight are needed to induce acute toxicity. Some [[pollution and pelagic fishes|fish]] and marine invertebrate [[species ]] experience acute toxic effects at diuron concentrations above 4,3 mg/l and 1 mg/l respectively<ref>[http://extoxnet.orst.edu/pips/diuron.htm www.extoxnet.orst.edu august 17 2009]</ref>. Algae are most vulnerable to the herbicide, it can be toxic for some algae species at concentrations of only 5 µg/l<ref>[http://www.pesticideinfo.org/List_AquireAcuteSum.jsp?Rec_Id=PC33293&Taxa_Group=Phytoplankton www.pesticideinfo.org august 17 2009]</ref>. |
<P> | <P> | ||
Revision as of 08:02, 5 October 2009
Definition of diruon:
Diuron is used as a herbicide on a variety of both crop and non-crop areas. It is also used as a mildewcide in paints and stains, and as an algaecide in commercial fish production[1].
This is the common definition for diruon, other definitions can be discussed in the article
|
Notes
| Diuron |
|---|
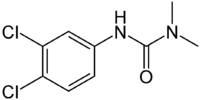
|
| Formula |
| C9H10Cl12N2O |
In the USA diuron has been used since 1967. Durion is mainly used on citrus, berries, asparagus and pineapple. Right-of-way applications (e.g., on rail road tracks) form the greatest non-agricultural use of diuron, with approximately 1 million kilograms applied annually.
In water durion has a low tendency to adsorb to particles and organic matter, dispite its relatively low water solubility of 42 mg/l. It's a stable molecule in terrestrial systems but can be biodegraded by micro-organisms. It is expected to be much less stable in aquatic systems, with a half-life of 30 days (according to lab experiments). Although it has a low potential towards bioaccumulation, it probably doesn't bioaccumulate or biomagnify in wild aquatic populations[2].
It has a low toxicity for mammals, as doses above 3,4 g per kg body weight are needed to induce acute toxicity. Some fish and marine invertebrate species experience acute toxic effects at diuron concentrations above 4,3 mg/l and 1 mg/l respectively[3]. Algae are most vulnerable to the herbicide, it can be toxic for some algae species at concentrations of only 5 µg/l[4].
Environmental standards and legislation
Included in the water framework list of priority substances
See also
Diuron on the ED North Database