Difference between revisions of "Diosgenin"
| Line 17: | Line 17: | ||
Diosgenin is made by hydrolysis of saponins, which are extracted from ''Dioscorea villosa'', a plant which grows in North America. It is also present in a number of other plant [[species]] which mainly occur in North America. | Diosgenin is made by hydrolysis of saponins, which are extracted from ''Dioscorea villosa'', a plant which grows in North America. It is also present in a number of other plant [[species]] which mainly occur in North America. | ||
<ref name="wiki">[http://en.wikipedia.org/wiki/Diosgenin http://en.wikipedia.org/wiki/Diosgenin]</ref>. | <ref name="wiki">[http://en.wikipedia.org/wiki/Diosgenin http://en.wikipedia.org/wiki/Diosgenin]</ref>. | ||
| − | Extracts of ''Dioscorea villosa'' containing diosgenin are sold in a variety of pharmacy and health food stores because of their presumed ability to minimize post-menopausal symptoms. <ref>[http://www.raysahelian.com/diosgenin.html www.raysahelian.com]</ref> It is also thought to induce apoptosis in cancer cells, and to reduce high blood pressure. <ref>[http://www.find-health-articles.com/rec_pub_18071250-antioxidative-hypolipidemic-effects-diosgenin-steroidal-saponin-yam.htm Son IS, Kim JH, Sohn HY, Son KH, Kim JS, Kwon CS. 2007 Antioxidative and hypolipidemic effects of diosgenin, a steroidal saponin of yam (Dioscorea spp.), on high-cholesterol fed rats. Biosci Biotechnol Biochem. 2007 Dec;71(12):3063-71. Epub 2007 Dec 7.]</ref> <ref name="an">[http://cebp.aacrjournals.org/cgi/content/full/13/8/1392 Jayadev Raju, Jagan M.R. Patlolla, Malisetty V. Swamy and Chinthalapally V. Rao 2004 Diosgenin, a Steroid Saponin of Trigonella foenum graecum (Fenugreek), Inhibits Azoxymethane-Induced Aberrant Crypt Foci Formation in F344 Rats and Induces Apoptosis in HT-29 Human Colon Cancer Cells Cancer Epidemiology Biomarkers & Prevention Vol. 13, 1392-1398, August 2004]</ref> | + | Extracts of ''Dioscorea villosa'' containing diosgenin are sold in a variety of pharmacy and health food stores because of their presumed ability to minimize post-menopausal symptoms. <ref>[http://www.raysahelian.com/diosgenin.html www.raysahelian.com August 12 2009]</ref> It is also thought to induce apoptosis in cancer cells, and to reduce high blood pressure. <ref>[http://www.find-health-articles.com/rec_pub_18071250-antioxidative-hypolipidemic-effects-diosgenin-steroidal-saponin-yam.htm Son IS, Kim JH, Sohn HY, Son KH, Kim JS, Kwon CS. 2007 Antioxidative and hypolipidemic effects of diosgenin, a steroidal saponin of yam (Dioscorea spp.), on high-cholesterol fed rats. Biosci Biotechnol Biochem. 2007 Dec;71(12):3063-71. Epub 2007 Dec 7.]</ref> <ref name="an">[http://cebp.aacrjournals.org/cgi/content/full/13/8/1392 Jayadev Raju, Jagan M.R. Patlolla, Malisetty V. Swamy and Chinthalapally V. Rao 2004 Diosgenin, a Steroid Saponin of Trigonella foenum graecum (Fenugreek), Inhibits Azoxymethane-Induced Aberrant Crypt Foci Formation in F344 Rats and Induces Apoptosis in HT-29 Human Colon Cancer Cells Cancer Epidemiology Biomarkers & Prevention Vol. 13, 1392-1398, August 2004]</ref> |
It has a very low water solubility 0.02 mg/l, is very hydrophobic is therefore in the marine environment expected to be mostly associated to organic matter, particles and sediments. It is also expected to have a high potential towards [[bioaccumulation]]. <ref>[http://old.iupac.org/publications/pac/2003/pdf/7511x1933.pdf Pim de Voogt and Bert van Hattum 2003 Critical factors in exposure modelling of endocrine active substances Pure Appl. Chem., Vol. 75, Nos. 11–12, pp. 1933–1948, 2003]</ref> | It has a very low water solubility 0.02 mg/l, is very hydrophobic is therefore in the marine environment expected to be mostly associated to organic matter, particles and sediments. It is also expected to have a high potential towards [[bioaccumulation]]. <ref>[http://old.iupac.org/publications/pac/2003/pdf/7511x1933.pdf Pim de Voogt and Bert van Hattum 2003 Critical factors in exposure modelling of endocrine active substances Pure Appl. Chem., Vol. 75, Nos. 11–12, pp. 1933–1948, 2003]</ref> | ||
Revision as of 09:39, 28 August 2009
Definition of Diosgenin:
Diosgenin is a pharmaceutical used for the synthesis of cortisone, progesterone, and other steroid products.[1]
This is the common definition for Diosgenin, other definitions can be discussed in the article
|
Notes
| Diosgenin |
|---|
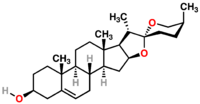
|
| Formula |
| C27H42O3 |
Diosgenin is made by hydrolysis of saponins, which are extracted from Dioscorea villosa, a plant which grows in North America. It is also present in a number of other plant species which mainly occur in North America. [1]. Extracts of Dioscorea villosa containing diosgenin are sold in a variety of pharmacy and health food stores because of their presumed ability to minimize post-menopausal symptoms. [2] It is also thought to induce apoptosis in cancer cells, and to reduce high blood pressure. [3] [4]
It has a very low water solubility 0.02 mg/l, is very hydrophobic is therefore in the marine environment expected to be mostly associated to organic matter, particles and sediments. It is also expected to have a high potential towards bioaccumulation. [5]
Environmental standards and legislation
Included in the OSPAR list of substances of priority action
References
- ↑ 1.0 1.1 www.wikipedia.org August 12 2009 Cite error: Invalid
<ref>tag; name "wiki" defined multiple times with different content - ↑ www.raysahelian.com August 12 2009
- ↑ Son IS, Kim JH, Sohn HY, Son KH, Kim JS, Kwon CS. 2007 Antioxidative and hypolipidemic effects of diosgenin, a steroidal saponin of yam (Dioscorea spp.), on high-cholesterol fed rats. Biosci Biotechnol Biochem. 2007 Dec;71(12):3063-71. Epub 2007 Dec 7.
- ↑ Jayadev Raju, Jagan M.R. Patlolla, Malisetty V. Swamy and Chinthalapally V. Rao 2004 Diosgenin, a Steroid Saponin of Trigonella foenum graecum (Fenugreek), Inhibits Azoxymethane-Induced Aberrant Crypt Foci Formation in F344 Rats and Induces Apoptosis in HT-29 Human Colon Cancer Cells Cancer Epidemiology Biomarkers & Prevention Vol. 13, 1392-1398, August 2004
- ↑ Pim de Voogt and Bert van Hattum 2003 Critical factors in exposure modelling of endocrine active substances Pure Appl. Chem., Vol. 75, Nos. 11–12, pp. 1933–1948, 2003