Difference between revisions of "Bisphenol-A"
(→Notes) |
|||
| Line 20: | Line 20: | ||
The total amount of bisphenol-A manufactured in 2005 within the EU was estimated at approximately 1.150.000 tonnes. This large amount was mainly produced to manufacture polycarbonate, witch is a widely used plastic.<ref name="echa">[http://echa.europa.eu/doc/trd_substances/4_4_isopropylidene_diphenol_bisphenol_a/ann_xv_trd/trd_uk_bisphenol_a.pdf ECHA 2008 ANNEX XV RESTRICTION REPORT Bisphenol-A]</ref> | The total amount of bisphenol-A manufactured in 2005 within the EU was estimated at approximately 1.150.000 tonnes. This large amount was mainly produced to manufacture polycarbonate, witch is a widely used plastic.<ref name="echa">[http://echa.europa.eu/doc/trd_substances/4_4_isopropylidene_diphenol_bisphenol_a/ann_xv_trd/trd_uk_bisphenol_a.pdf ECHA 2008 ANNEX XV RESTRICTION REPORT Bisphenol-A]</ref> | ||
| − | In water bisphenol A is moderately soluble, 300 mg/l, and considered to have a moderate tendency to [[adsorption|adsorb]] to suspended particles and sediments. It has a very low tendency to evaporate into the atmosphere where most of it will be degraded in less than a day. In water and soils it is rather stable, although it can readily be biodegraded. The environmental [[half- | + | In water bisphenol A is moderately soluble, 300 mg/l, and considered to have a moderate tendency to [[adsorption|adsorb]] to suspended particles and sediments. It has a very low tendency to evaporate into the atmosphere where most of it will be degraded in less than a day. In water and soils it is rather stable, although it can readily be biodegraded. The environmental [[half-life]] is only 3 to 8 days and a 100% removal of environmental contamination can occur within 17 days. In [[anoxic]] sediments bisphenol A can be created from the degradation of [[Tetrabromobisphenol A|TBBP-A]].<ref name="en">[http://ecb.jrc.it/documents/Existing-Chemicals/RISK_ASSESSMENT/ADDENDUM/bisphenola_add_325.pdf February 2008 Updated European Risk Assessment Report 4,4’-ISOPROPYLIDENEDIPHENOL (BISPHENOL-A)]</ref> |
| − | Bisphenol A has a low tendency to [[bioaccumulation|bioaccumulate]]. Therefore in poses a low toxicity threat by biomagnification towards marine mammals. | + | Bisphenol A has a low tendency to [[bioaccumulation|bioaccumulate]]. Therefore in poses a low toxicity threat by [[biomagnification]] towards marine mammals. Bisphenol A become lethal when mammals consume each day more than 33 mg of it per kg of body weight.<ref name="en">[http://ecb.jrc.it/documents/Existing-Chemicals/RISK_ASSESSMENT/ADDENDUM/bisphenola_add_325.pdf February 2008 Updated European Risk Assessment Report 4,4’-ISOPROPYLIDENEDIPHENOL (BISPHENOL-A)]</ref> |
| − | Bisphenol | + | Bisphenol A exhibits [[endocrine disrupting compounds|endocrine disrupting effects]]. In [http://www.marinespecies.org/aphia.php?p=taxdetails&id=101 gastropods] concentrations bellow 100 µg/l have been shown cause reduced penis sizes in males and enhanced oocyte production in females. The latter results in an increased embryo production at low bisphenol A concentrations. The latter effect has even been demonstrated in some gastropod species at concentrations of only 100 ng/l. <ref name="echa">[http://echa.europa.eu/doc/trd_substances/4_4_isopropylidene_diphenol_bisphenol_a/ann_xv_trd/trd_uk_bisphenol_a.pdf ECHA 2008 ANNEX XV RESTRICTION REPORT Bisphenol-A]</ref> It demonstrates a moderate acute toxicity towards aquatic species. Most species start dying at concentrations above 1 mg/l. <ref name="pe">[http://www.pesticideinfo.org/Detail_Chemical.jsp?Rec_Id=PC33756#Related_Chems www.pesticideinfo.org August 24 2009]</ref> |
<P> | <P> | ||
Revision as of 10:29, 27 August 2009
Definition of Bisphenol-A:
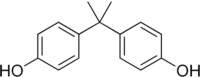
Bisphenol-A is also known as 4,4’-Isopropylidenediphenol. At room temperature it occurs as a white powder or in flakes. [1]
This is the common definition for Bisphenol-A, other definitions can be discussed in the article
|
Notes
| Bisphenol-A |
|---|
|
| Formula |
| C15H16O2 |
The total amount of bisphenol-A manufactured in 2005 within the EU was estimated at approximately 1.150.000 tonnes. This large amount was mainly produced to manufacture polycarbonate, witch is a widely used plastic.[2]
In water bisphenol A is moderately soluble, 300 mg/l, and considered to have a moderate tendency to adsorb to suspended particles and sediments. It has a very low tendency to evaporate into the atmosphere where most of it will be degraded in less than a day. In water and soils it is rather stable, although it can readily be biodegraded. The environmental half-life is only 3 to 8 days and a 100% removal of environmental contamination can occur within 17 days. In anoxic sediments bisphenol A can be created from the degradation of TBBP-A.[1]
Bisphenol A has a low tendency to bioaccumulate. Therefore in poses a low toxicity threat by biomagnification towards marine mammals. Bisphenol A become lethal when mammals consume each day more than 33 mg of it per kg of body weight.[1]
Bisphenol A exhibits endocrine disrupting effects. In gastropods concentrations bellow 100 µg/l have been shown cause reduced penis sizes in males and enhanced oocyte production in females. The latter results in an increased embryo production at low bisphenol A concentrations. The latter effect has even been demonstrated in some gastropod species at concentrations of only 100 ng/l. [2] It demonstrates a moderate acute toxicity towards aquatic species. Most species start dying at concentrations above 1 mg/l. [3]
Environmental standards and legislation
Included in the water framework list of priority substances
See also
Bisphenol-A on ED North Database