Difference between revisions of "AMPA"
From Coastal Wiki
| Line 1: | Line 1: | ||
{{Definition|title=Aminomethylphosphonic acid (AMPA) | {{Definition|title=Aminomethylphosphonic acid (AMPA) | ||
| − | |definition=Aminomethylphosphonic acid is mainly produced by environmental biodegradation reactions of the herbicide glyphosate. <ref>[http://www.cdpr.ca.gov/docs/emon/pubs/fatememo/glyphos.pdf Canadian Department of Pesticide Regulation 1998 ENVIRONMENTAL FATE OF GLYPHOSATE]</ref>}} | + | |definition=Aminomethylphosphonic acid is mainly produced by environmental biodegradation reactions of the herbicide [[glyphosate]]. <ref>[http://www.cdpr.ca.gov/docs/emon/pubs/fatememo/glyphos.pdf Canadian Department of Pesticide Regulation 1998 ENVIRONMENTAL FATE OF GLYPHOSATE]</ref>}} |
Revision as of 14:48, 24 August 2009
Definition of Aminomethylphosphonic acid (AMPA):
Aminomethylphosphonic acid is mainly produced by environmental biodegradation reactions of the herbicide glyphosate. [1]
This is the common definition for Aminomethylphosphonic acid (AMPA), other definitions can be discussed in the article
|
Notes
| AMPA |
|---|
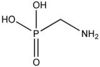
|
| Formula |
| CH6NO3P |
Currently very little is known about this AMPA. It is formed by biodegradation of the herbicide glyohosate and can be found at lower concentrations in the environment. It adsorbs more strongly to soils than glysphosate and might have a higher tendency towards bioaccumulation. Glysphosate however is not expected to bioaccumulate much because of its high water solubility. AMPA is also more stable than glyphosate. [2]
Environmental standards and legislation
Included in the water framework list of priority substances