Difference between revisions of "C10-13-cloroalkanes"
| Line 1: | Line 1: | ||
{{ Definition|title= C<sub>10-13</sub>-chloroalkanes | {{ Definition|title= C<sub>10-13</sub>-chloroalkanes | ||
| − | |definition=C<sub>10-13</sub>-chloroalkanes are UVCB substances (Substances of Unknown or Variable Composition | + | |definition=C<sub>10-13</sub>-chloroalkanes are UVCB substances (Substances of Unknown or Variable Composition) with varying chlorine contents (up to around 70% by weight) and carbon chain lengths (between C10 and C13). <ref name="echa">[http://echa.europa.eu/doc/candidate_list/svhc_supdoc_alkanes_c10_13_chloro_publication.pdf European chemicals agency 2008 MEMBER STATE COMMITTEE SUPPORT DOCUMENT FOR IDENTIFICATION OF ALKANES, C10-13, CHLORO AS A SUBSTANCE OF VERY HIGH CONCERN]</ref> They are yellowish oily liquids, runny or thick, without a distinct melting point, but undergo a process of thickening at temperatures below 35 °C. <ref name="pollu">[http://pollution.unibuc.ro/?substance=28 http://pollution.unibuc.ro/ 19 August 2009]</ref> }} |
== Notes == | == Notes == | ||
Revision as of 10:32, 27 August 2009
Definition of C10-13-chloroalkanes:
C10-13-chloroalkanes are UVCB substances (Substances of Unknown or Variable Composition) with varying chlorine contents (up to around 70% by weight) and carbon chain lengths (between C10 and C13). [1] They are yellowish oily liquids, runny or thick, without a distinct melting point, but undergo a process of thickening at temperatures below 35 °C. [2]
This is the common definition for C10-13-chloroalkanes, other definitions can be discussed in the article
|
Notes
| C10- and C13-chloroalkane |
|---|
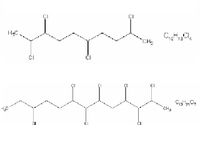
|
| Formula |
| C10H18Cl4 and C13H21Cl7 |
The main use was as industrial metal working fluids used in processes involving cutting, drilling, machining and stamping metal in engineering and manufacturing. Because of their fire retarding properties, C10-13-chloroalkanes are used as an additive in rubber, especially for conveyor belts. They are also used in paints and other coatings, leathers, textiles and in sealing compounds. Their use in metal working fluids and for fat liquoring of leather is prohibited in the EU by January 2004 (for use as substances or as constituents of other substances or preparations in concentrations higher than 1%). C10-13-chloroalkanes were mainly released to water as a result of fugitive emissions during manufacture and use as metal working fluids. Accidental releases during transport and storage may also result in releases. They have a low volatility which causes only limited amounts to be evaporated into the atmosphere where they would be degraded rather rapidly. [2] [1]
C10-13-chloroalkanes have a very low water solubility (between 0,15 and 0,47 mg/l) and a strong tendency to adsorb to organic matter and soils. In marine waters they are not considered to be easily degraded, and can be very persistent in the soils where it can take more than 450 days to half its concentration.
The substance has a high bioaccumulation potential. This is confirmed by experiments with fish which could accumulate concentrations up to 8000 times higher than those in the surrounding water. Bioaccumulation has also been confirmed in other species such as molluscs. Therefore C10-13-chloroalkanes is considered likely to biomagnify through food chains.[1]
C10-13-chloroalkanes are very toxic to zooplankton. Prolonged exposure to concentrations above 16,3 µg/l have been shown to cause mortalities to a water fleas. Fishes are more tolerant and only start dying by prolonged exposure to concentrations above 300 µg/l. Because of the its potential to biomagnify C10-13-chloroalkanes could pose threats of secondary poisoning towards fish eating sea birds and marine mammals. Prolonged exposure to doses of 100 mg of C10-13-chloroalkanes per kilogram of body weight a day has been shown to affect the livers and kidneys of rats. [1]
Environmental standards and legislation
Included in the water framework list of priority substances