Difference between revisions of "Pentachlorophenol"
| Line 2: | Line 2: | ||
{{Definition|title=pentachlorophenol (PCP) | {{Definition|title=pentachlorophenol (PCP) | ||
| − | |definition= Pentachlorophenol is a white organic solid with needle-like crystals and a phenolic | + | |definition= Pentachlorophenol is a white organic solid with needle-like crystals and a phenolic odour<ref name = epa>[http://www.epa.gov/safewater/contaminants/dw_contamfs/pentachl.html]</ref>. It is an [[organochlorine compounds|organochlorine compound]] used mainly as a fungicide. Sodium pentachlorophenate and pentachlorophenyl laurate (PCPL), are used for similar purposes<ref name = OECD>[http://www.ospar.org/documents%5Cdbase%5Cpublications%5Cp00138_BD%20on%20pentachlorophenol.pdf OSPAR Commission, 2004: OSPAR background document on pentachlorophenol]</ref>.}} |
== Notes == | == Notes == | ||
Revision as of 10:11, 31 August 2009
Definition of pentachlorophenol (PCP):
Pentachlorophenol is a white organic solid with needle-like crystals and a phenolic odour[1]. It is an organochlorine compound used mainly as a fungicide. Sodium pentachlorophenate and pentachlorophenyl laurate (PCPL), are used for similar purposes[2].
This is the common definition for pentachlorophenol (PCP), other definitions can be discussed in the article
|
Notes
| Pentachlorophenol |
|---|
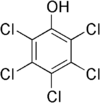
|
| Formula |
| C6HCl5O |
Production of PCP in the EU ceased in 1992. In 1996, 378 tonnes of NaPCP and 30 tonnes of PCP were imported from the USA; there may possibly be other imports from Asia. NaPCP was mainly used in France, Portugal and Spain, as a control agent to protect newly cut wood surfaces against fungal attack. Treatment of wood and textiles resulted in release of PCP to the environment. Since 2008 no products which contain more than 0,1% of PCP can be sold.
Typical concentrations of PCP in the North Sea are around 0,07 µg/l, with a maximum detected concentration of 0,79 µg/l. These concentrations have been decreasing since 1983. [2]
Due to it's low water solubility PCP in the the marine environment will adsorb to sediments, where it is quite stable. In water it can be degraded by photolysis.[1] PCP has a tendency to bioacumulate and biomagnify.[3] It also is considered toxic for marine organisms. In humans it can cause liver damage and have endocrine disrupting effects[4]
Its main degradation product is pentachloroanisole.
Environmental standards and legislation
Included in the OSPAR list of substances of priority action
Included in the water framework list of priority substances
See also
Pentachlorophenol on the ED North Database
OSPAR background document on pentachlorophenol