Difference between revisions of "Pentachlorobenzene"
Dronkers J (talk | contribs) |
|||
| (One intermediate revision by one other user not shown) | |||
| Line 21: | Line 21: | ||
PeCB is stable under environmental conditions, it mainly disappears from water bodies and soils by evaporation, which is rather low. In fact in equilibrium conditions 30,6% of the environmental PeCB is expected to be in the atmosphere 68,1% [[adsorption|adsorbed]] to soils, 0,2% dissolved in water bodies and the remaining 1,1% adsorbed to sediments. Atmospherical PeCB is faster degraded than that in the soil or in water although it still has a [[half-life]] of 170 to 300 days. PeCB can therefore be transported globally. | PeCB is stable under environmental conditions, it mainly disappears from water bodies and soils by evaporation, which is rather low. In fact in equilibrium conditions 30,6% of the environmental PeCB is expected to be in the atmosphere 68,1% [[adsorption|adsorbed]] to soils, 0,2% dissolved in water bodies and the remaining 1,1% adsorbed to sediments. Atmospherical PeCB is faster degraded than that in the soil or in water although it still has a [[half-life]] of 170 to 300 days. PeCB can therefore be transported globally. | ||
| − | PeCB demonstrates a high [[bioaccumulation]] potential in [[pollution and pelagic fishes|fishes]]. A variety of studies showed that fish tissues accumulate to concentrations which are between 6.500 and 13.000 times higher than those of their environment. There appears to be however quite little [[biomagnification]] in aquatic | + | PeCB demonstrates a high [[bioaccumulation]] potential in [[pollution and pelagic fishes|fishes]]. A variety of studies showed that fish tissues accumulate to concentrations which are between 6.500 and 13.000 times higher than those of their environment. There appears to be however quite little [[biomagnification]] in aquatic food webs<ref name="ch">[http://www.eurochlor.org/upload/documents/document260.pdf Eurochlor 2007 Pentachlorobenzene – Sources, environmental fate and risk characterization]</ref>. |
Chronic [[toxic|toxicity]] can be caused to some fishes species by exposure to concentrations above 50 µg/l. One crab species dies when exposed during moulting to concentrations of 75 µg/l, although some other marine invertebrate species were able to tolerate short exposure to concentrations up to 300µg/l and some even above 3 mg/l. | Chronic [[toxic|toxicity]] can be caused to some fishes species by exposure to concentrations above 50 µg/l. One crab species dies when exposed during moulting to concentrations of 75 µg/l, although some other marine invertebrate species were able to tolerate short exposure to concentrations up to 300µg/l and some even above 3 mg/l. | ||
| Line 38: | Line 38: | ||
==References== | ==References== | ||
<references/> | <references/> | ||
| + | |||
{{author | {{author | ||
|AuthorID=19826 | |AuthorID=19826 | ||
|AuthorFullName=Daphnis De Pooter | |AuthorFullName=Daphnis De Pooter | ||
|AuthorName=Daphnisd}} | |AuthorName=Daphnisd}} | ||
| − | [[Category: | + | |
| + | [[Category:Toxicity chemicals]] | ||
Latest revision as of 13:32, 9 August 2020
Definition of pentachlorobenzene PeCB:
Pentachlorobenzene occurs as white or colourless crystals and has an odour. In the past, PeCB was one component of a chlorobenzene mixture used to reduce the viscosity of PCB products employed for heat transfer. PeCB has also been used in a chlorobenzenes mixture with PCBs in electrical equipment[1] [2].
This is the common definition for pentachlorobenzene PeCB, other definitions can be discussed in the article
|
Notes
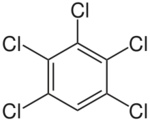
| Pentachlorobenzene (PeCB) |
|---|
|
| Formula |
| C6HCl5 |
There are no large scale uses of PeCB at present. Current emissions of PeCB to the environment are estimated to be about 85.000 kg/year. The largest sources appear to be combustion of solid wastes (33.000 kg/year) and biomass burning (44.000 kg/year)[2].
PeCB is stable under environmental conditions, it mainly disappears from water bodies and soils by evaporation, which is rather low. In fact in equilibrium conditions 30,6% of the environmental PeCB is expected to be in the atmosphere 68,1% adsorbed to soils, 0,2% dissolved in water bodies and the remaining 1,1% adsorbed to sediments. Atmospherical PeCB is faster degraded than that in the soil or in water although it still has a half-life of 170 to 300 days. PeCB can therefore be transported globally.
PeCB demonstrates a high bioaccumulation potential in fishes. A variety of studies showed that fish tissues accumulate to concentrations which are between 6.500 and 13.000 times higher than those of their environment. There appears to be however quite little biomagnification in aquatic food webs[2].
Chronic toxicity can be caused to some fishes species by exposure to concentrations above 50 µg/l. One crab species dies when exposed during moulting to concentrations of 75 µg/l, although some other marine invertebrate species were able to tolerate short exposure to concentrations up to 300µg/l and some even above 3 mg/l.
Concentrations in the Pacific Ocean average at 16 pg/l[2].
Environmental standards and legislation
Included in the water framework list of priority substances
References
Please note that others may also have edited the contents of this article.
|