Difference between revisions of "Dicofol"
Dronkers J (talk | contribs) |
|||
| (7 intermediate revisions by one other user not shown) | |||
| Line 1: | Line 1: | ||
| − | + | {{Tocright}} | |
{{Definition|title=dicofol | {{Definition|title=dicofol | ||
| − | |definition=Dicofol belongs to the group of [[organochlorine pesticides]]. It's produced from [[DDT]], therefore it has a | + | |definition=Dicofol belongs to the group of [[organochlorine pesticides]]. It's produced from [[DDT]], therefore it has a similar chemical structure and similar properties<ref name="Ospar">[http://www.ospar.org/documents%5Cdbase%5Cpublications%5Cp00150_Background%20document%20on%20Dicofol.pdf OSPAR Commission, 2004: OSPAR background document on dicofol]</ref>.}} |
== Notes == | == Notes == | ||
| − | |||
{| class="toccolours" border="1" style="float: right; clear: right; margin: 0 0 1em 1em; border-collapse: collapse;" | {| class="toccolours" border="1" style="float: right; clear: right; margin: 0 0 1em 1em; border-collapse: collapse;" | ||
| Line 18: | Line 17: | ||
|} | |} | ||
| − | + | The main source of dicofol in the environment is its use as a [[pesticide]] used on a wide variety of fruits, vegetables, ornamentals and field crops. A total amount of over 2700 tonnes of dicofol is used around the world each year, of which 290 tonnes is used in Western Europe. The only European countries which do allow its use are Belgium, Spain, Portugal and France. There are indications that through atmospheric transport, dicophol used in other continents might also end up in the [[North Sea]]. <ref name="Ospar">[http://www.ospar.org/documents%5Cdbase%5Cpublications%5Cp00150_Background%20document%20on%20Dicofol.pdf OSPAR Commission, 2004: OSPAR background document on dicofol]</ref> | |
| − | The main source of dicofol in the environment is its use as a [[pesticide]] used on a wide variety of fruits, vegetables, ornamentals and field crops. A total amount of over 2700 tonnes of dicofol is used around the world each year, of which 290 tonnes is used in Western Europe. The only European countries which do allow its use are Belgium, Spain, Portugal and France. There are indications that through atmospheric transport dicophol used in other continents might also end up in the North Sea. <ref name="Ospar">[http://www.ospar.org/documents%5Cdbase%5Cpublications%5Cp00150_Background%20document%20on%20Dicofol.pdf OSPAR Commission, 2004: OSPAR background document on dicofol]</ref> | ||
The degradation of dicofol in soil is moderately slow, with a [[half-life]] of 30 to 60 days. In water systems and soils with a high organic matter content, the half-life can reach 84 days. Like DDT, it also has a high affinity to lipids, which causes it to [[adsorption|adsob]] to organic particles rather than dissolve in water. It's also highly [[bioaccumulation|bioaccumulative]], and [[biomagnification|biomagnifying]], therefore it poses a greater threat to high [[trophic level]] [[species]] such as [[pollution and sea birds|birds]] and [[pollution and marine mammals|mammals]]. | The degradation of dicofol in soil is moderately slow, with a [[half-life]] of 30 to 60 days. In water systems and soils with a high organic matter content, the half-life can reach 84 days. Like DDT, it also has a high affinity to lipids, which causes it to [[adsorption|adsob]] to organic particles rather than dissolve in water. It's also highly [[bioaccumulation|bioaccumulative]], and [[biomagnification|biomagnifying]], therefore it poses a greater threat to high [[trophic level]] [[species]] such as [[pollution and sea birds|birds]] and [[pollution and marine mammals|mammals]]. | ||
| − | Dicofol has been shown to be highly toxic for marine species. [[Pollution and pelagic fishes| | + | Dicofol has been shown to be highly [[toxic]] for marine species. [[Pollution and pelagic fishes|Fishes]] can die by chronic exposure to concentrations above 4,5 µg/l and concentrations above 12 µg/l can cause acute toxicity. |
| − | In birds | + | In birds exposure to 20 mg of dicofol per kg body weight can cause eggshell thinning. It has also been shown to be a [[endocrine disrupting compounds|endocrine disrupting compound]]: male juvenile birds with a daily food intake of 5 mg dicofol per kg body weight showed effects of feminisation, which affected their mating behaviour and reproduction success<ref name="Ospar">[http://www.ospar.org/documents%5Cdbase%5Cpublications%5Cp00150_Background%20document%20on%20Dicofol.pdf OSPAR Commission, 2004: OSPAR background document on dicofol]</ref>. |
<P> | <P> | ||
<BR> | <BR> | ||
| Line 49: | Line 47: | ||
==References== | ==References== | ||
<references/> | <references/> | ||
| + | {{author | ||
| + | |AuthorID=19826 | ||
| + | |AuthorFullName=Daphnis De Pooter | ||
| + | |AuthorName=Daphnisd}} | ||
| − | [[Category: | + | [[Category:Toxicity chemicals]] |
Latest revision as of 13:08, 9 August 2020
Definition of dicofol:
Dicofol belongs to the group of organochlorine pesticides. It's produced from DDT, therefore it has a similar chemical structure and similar properties[1].
This is the common definition for dicofol, other definitions can be discussed in the article
|
Notes
| Dicofol |
|---|
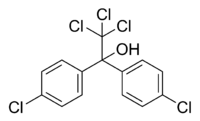
|
| Formula |
| C14H9Cl5O |
The main source of dicofol in the environment is its use as a pesticide used on a wide variety of fruits, vegetables, ornamentals and field crops. A total amount of over 2700 tonnes of dicofol is used around the world each year, of which 290 tonnes is used in Western Europe. The only European countries which do allow its use are Belgium, Spain, Portugal and France. There are indications that through atmospheric transport, dicophol used in other continents might also end up in the North Sea. [1]
The degradation of dicofol in soil is moderately slow, with a half-life of 30 to 60 days. In water systems and soils with a high organic matter content, the half-life can reach 84 days. Like DDT, it also has a high affinity to lipids, which causes it to adsob to organic particles rather than dissolve in water. It's also highly bioaccumulative, and biomagnifying, therefore it poses a greater threat to high trophic level species such as birds and mammals.
Dicofol has been shown to be highly toxic for marine species. Fishes can die by chronic exposure to concentrations above 4,5 µg/l and concentrations above 12 µg/l can cause acute toxicity. In birds exposure to 20 mg of dicofol per kg body weight can cause eggshell thinning. It has also been shown to be a endocrine disrupting compound: male juvenile birds with a daily food intake of 5 mg dicofol per kg body weight showed effects of feminisation, which affected their mating behaviour and reproduction success[1].
Environmental standards and legislation
Included in the OSPAR list of substances of priority action
Included in the water framework list of priority substances
See also
Dicofol on the ED North Database
OSPAR background document on dicofol
References
Please note that others may also have edited the contents of this article.
|