Difference between revisions of "1,2-Dichloroethane"
Dronkers J (talk | contribs) |
|||
| (19 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
| + | {{Tocright | ||
| + | }} | ||
{{Definition|title=1,2-dichloroethane | {{Definition|title=1,2-dichloroethane | ||
| − | + | |definition=1,2-Dichloroethane is a clear, chemically manufactured liquid. It evaporates quickly at room temperature and has a pleasant smell and a sweet taste. The most common use of 1,2-dichloroethane is the formation of vinyl chloride, used in the production of a variety of plastic and vinyl products. These include important construction materials such as polyvinyl chloride (PVC) pipes, but also packaging materials, furniture, auto mobile parts, wall coverings and housewares<ref name="US">[http://www.atsdr.cdc.gov/toxprofiles/tp38.pdf U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES 2001 TOXICOLOGICAL PROFILE FOR 1,2-DICHLOROETHANE]</ref>. }} | |
| − | |definition=1,2-Dichloroethane is a clear chemically manufactured liquid. It evaporates quickly at room temperature and has a pleasant smell and a sweet taste. The most common use of 1,2-dichloroethane is | ||
== Notes == | == Notes == | ||
| Line 16: | Line 17: | ||
|} | |} | ||
| − | US annual production of 1,2-dichloroethane averaged around 7 million tonnes in the 1990s. It can enter the environment during manufacture, transport or use. | + | US annual production of 1,2-dichloroethane averaged around 7 million tonnes in the 1990s. It can enter the environment during manufacture, transport or use. 1,2-dichloroethane is mostly released to the air, although some is released to rivers or lakes<ref name="US"/>. |
| − | |||
| − | |||
| − | |||
| − | + | 1,2-Dichloroethane can evaporate rapidly from water or soils to the atmosphere where it is slowly degraded. It can [[persistent|persist]] in the atmosphere, with a [[half-life]] of 5 months, and during which it can be transported over large distances. It has a moderate water solubility of 8,69 g/l and is not expected to [[adsorption|adsorb]] to particles or sediments. In water it is slowly degraded, almost not biodegraded and removal mainly occurs by evaporation: The half-life of 1,2-dichloroethane in water is 10 days. <P> | |
| + | 1,2-Dichloroethane doesn't have a tendency to [[bioaccumulation|bioaccumulate]] and is therefore not expected to [[biomagnification|biomagnify]] through [[food chain|food chains]]<ref name="US"/> | ||
| + | Concentrations of 1,2-dichloroethane above 118 mg/l cause acute [[toxic|toxicity]] to marine fish, concentrations above 30 mg/l, 36 mg/l and 100 mg/l cause acute toxicity to respectively fresh water fish, marine invertebrates and marine algae<ref name = chl>De Rooij, C., Boutonnet, J., Garny, V. et al. Euro Chlor Risk Assessment for the Marine Environment Osparcom Region: North Sea - 1,2-Dichloroethane. Environ Monit Assess 53, 425–445 (1998)</ref>. | ||
| − | It is suspected that 1,2-dichloroethane in the North Sea might reach concentrations up to 6,4 µg/l | + | It is suspected that 1,2-dichloroethane in heavily [[pollution|polluted]] [[coastal area|coastal areas]] of the [[North Sea]] might reach concentrations up to 6,4 µg/l. Typical concentrations in polluted [[estuary|estuaries]] range around 0,5 µg/l and those in [[Open oceans|open seas]] around 0.005 µg/l<ref name = chl/>. |
<P> | <P> | ||
<BR> | <BR> | ||
| Line 34: | Line 34: | ||
<BR> | <BR> | ||
<P> | <P> | ||
| + | {{author | ||
| + | |AuthorID=19826 | ||
| + | |AuthorFullName=Daphnis De Pooter | ||
| + | |AuthorName=Daphnisd}} | ||
==References== | ==References== | ||
<references/> | <references/> | ||
| − | [[Category: | + | [[Category:Toxicity chemicals]] |
Latest revision as of 12:52, 9 August 2020
Definition of 1,2-dichloroethane:
1,2-Dichloroethane is a clear, chemically manufactured liquid. It evaporates quickly at room temperature and has a pleasant smell and a sweet taste. The most common use of 1,2-dichloroethane is the formation of vinyl chloride, used in the production of a variety of plastic and vinyl products. These include important construction materials such as polyvinyl chloride (PVC) pipes, but also packaging materials, furniture, auto mobile parts, wall coverings and housewares[1].
This is the common definition for 1,2-dichloroethane, other definitions can be discussed in the article
|
Notes
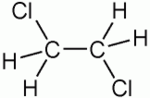
| 1,2-Dichloroethane |
|---|
|
| Formula |
| C2H4Cl2 |
US annual production of 1,2-dichloroethane averaged around 7 million tonnes in the 1990s. It can enter the environment during manufacture, transport or use. 1,2-dichloroethane is mostly released to the air, although some is released to rivers or lakes[1].
1,2-Dichloroethane can evaporate rapidly from water or soils to the atmosphere where it is slowly degraded. It can persist in the atmosphere, with a half-life of 5 months, and during which it can be transported over large distances. It has a moderate water solubility of 8,69 g/l and is not expected to adsorb to particles or sediments. In water it is slowly degraded, almost not biodegraded and removal mainly occurs by evaporation: The half-life of 1,2-dichloroethane in water is 10 days.1,2-Dichloroethane doesn't have a tendency to bioaccumulate and is therefore not expected to biomagnify through food chains[1] Concentrations of 1,2-dichloroethane above 118 mg/l cause acute toxicity to marine fish, concentrations above 30 mg/l, 36 mg/l and 100 mg/l cause acute toxicity to respectively fresh water fish, marine invertebrates and marine algae[2]. It is suspected that 1,2-dichloroethane in heavily polluted coastal areas of the North Sea might reach concentrations up to 6,4 µg/l. Typical concentrations in polluted estuaries range around 0,5 µg/l and those in open seas around 0.005 µg/l[2].
Environmental standards and legislation
Included in the water framework list of priority substances
Please note that others may also have edited the contents of this article.
|
References
- ↑ 1.0 1.1 1.2 U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES 2001 TOXICOLOGICAL PROFILE FOR 1,2-DICHLOROETHANE
- ↑ 2.0 2.1 De Rooij, C., Boutonnet, J., Garny, V. et al. Euro Chlor Risk Assessment for the Marine Environment Osparcom Region: North Sea - 1,2-Dichloroethane. Environ Monit Assess 53, 425–445 (1998)