Difference between revisions of "AMPA"
From Coastal Wiki
Dronkers J (talk | contribs) |
|||
| (7 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
| + | {{Tocright}} | ||
{{Definition|title=Aminomethylphosphonic acid (AMPA) | {{Definition|title=Aminomethylphosphonic acid (AMPA) | ||
| − | |definition=Aminomethylphosphonic acid is mainly produced by environmental biodegradation reactions of the herbicide [[glyphosate]] | + | |definition=Aminomethylphosphonic acid is mainly produced by environmental biodegradation reactions of the herbicide [[glyphosate]]<ref>[http://www.cdpr.ca.gov/docs/emon/pubs/fatememo/glyphos.pdf Canadian Department of Pesticide Regulation 1998 ENVIRONMENTAL FATE OF GLYPHOSATE]</ref>. }} |
| Line 17: | Line 18: | ||
|} | |} | ||
| − | Currently very little is known about | + | Currently very little is known about AMPA. It is formed by biodegradation of the herbicide glyohosate. Compared to glyohosate; present at lower concentrations in the environment, although it's more stable, it [[adsorption|adsorbs]] more strongly to soils and might have a higher tendency towards [[bioaccumulation]]. Glysphosate itself however is not expected to bioaccumulate much because of its higher water solubility<ref>[http://www.pesticideinfo.org/Detail_Chemical.jsp?Rec_Id=PC37655 www.pesticideinfo.org August 20 2009]</ref>. |
| − | Measured concentrations of AMPA in surface waters range between 6 and 35 µg/l <ref name= incham>[http://www.inchem.org/documents/ehc/ehc/ehc159.htm www.inchem.org August 25 2009.]</ref> | + | Measured concentrations of AMPA in surface waters range between 6 and 35 µg/l <ref name= incham>[http://www.inchem.org/documents/ehc/ehc/ehc159.htm www.inchem.org August 25 2009.]</ref>. |
<P> | <P> | ||
<BR> | <BR> | ||
<P> | <P> | ||
| + | |||
== Environmental standards and legislation == | == Environmental standards and legislation == | ||
| Line 32: | Line 34: | ||
==References== | ==References== | ||
<references/> | <references/> | ||
| + | {{author | ||
| + | |AuthorID=19826 | ||
| + | |AuthorFullName=Daphnis De Pooter | ||
| + | |AuthorName=Daphnisd}} | ||
| − | [[Category: | + | [[Category:Toxicity chemicals]] |
Latest revision as of 13:00, 9 August 2020
Definition of Aminomethylphosphonic acid (AMPA):
Aminomethylphosphonic acid is mainly produced by environmental biodegradation reactions of the herbicide glyphosate[1].
This is the common definition for Aminomethylphosphonic acid (AMPA), other definitions can be discussed in the article
|
Notes
| AMPA |
|---|
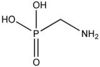
|
| Formula |
| CH6NO3P |
Currently very little is known about AMPA. It is formed by biodegradation of the herbicide glyohosate. Compared to glyohosate; present at lower concentrations in the environment, although it's more stable, it adsorbs more strongly to soils and might have a higher tendency towards bioaccumulation. Glysphosate itself however is not expected to bioaccumulate much because of its higher water solubility[2].
Measured concentrations of AMPA in surface waters range between 6 and 35 µg/l [3].
Environmental standards and legislation
Included in the water framework list of priority substances
References
Please note that others may also have edited the contents of this article.
|