Difference between revisions of "Fluoranthene"
Dronkers J (talk | contribs) |
|||
| (8 intermediate revisions by one other user not shown) | |||
| Line 1: | Line 1: | ||
| − | + | {{tocright}} | |
{{Definition|title=fluoranthene | {{Definition|title=fluoranthene | ||
| − | |definition=Fluoranthene is a [[PAH|polycyclic aromatic hydrocarbon]] (PAH). It exists as pale yellow needles or plates. Fluoranthene can be produced by the pyrolysis of organic raw materials such as coal and petroleum | + | |definition=Fluoranthene is a [[PAH|polycyclic aromatic hydrocarbon]] (PAH). It exists as pale yellow needles or plates. Fluoranthene can be produced by the pyrolysis at high temperatures of organic raw materials such as coal and petroleum. It is also known to to be produced by certain plants. Fluoranthene is a constituent of coal tar and petroleum-derived asphalt. Currently, there is no known production or use of this compound<ref name =RI>[http://rais.ornl.gov/tox/profiles/fluoran.shtml#t4 The Risk Assessment Information System Toxicity Summary for FLUORANTHENE August 17 2009]</ref>. }} |
== Notes == | == Notes == | ||
| Line 9: | Line 9: | ||
! bgcolor="#FF8888" | Fluoranthene | ! bgcolor="#FF8888" | Fluoranthene | ||
|- | |- | ||
| − | | align="center" bgcolor="#FFFFFF" | [[Image:Fluoranthene.svg.png| | + | | align="center" bgcolor="#FFFFFF" | [[Image:Fluoranthene.svg.png|125px|Fluoranthene]] |
|- | |- | ||
! bgcolor="#8888FF" | Formula | ! bgcolor="#8888FF" | Formula | ||
| Line 17: | Line 17: | ||
|} | |} | ||
| − | + | Since it is a universal product of combustion of organic matter and is present in fossil fuel products, fluoranthene is generally released into air and water. In the atmosphere fluoranthene is present both as vapour and [[adsorption|adsorbed]] to particles. As vapour it will be degraded rather rapidly, but it's more stable when adsorbed to particles and as such fluoranthene can travel large distances before being deposited. | |
| − | Fluoranthene has a low water solubility of 265 µg/l, and will | + | Fluoranthene has a low water solubility of 265 µg/l, and will rapidly be adsorbed to sediment and particulate matter. It disappears from the water column, by degradation and transport to the sediments. In the sediments however, it can be very stable for decades. |
| − | Since it has a high tendency to adsorb to organic matter, it has a high potential towards [[bioaccumulation]]. | + | Since it has a high tendency to adsorb to organic matter, it has a high potential towards [[bioaccumulation]]. It bioaccumulates in shellfish, making them an excellent [[bioindicator]] for fluoranthene [[pollution]]<ref>[http://www.speclab.com/compound/c206440.htm www.speclab.com August 17 2009]</ref>. |
| − | <ref>[http://www.speclab.com/compound/c206440.htm www.speclab.com 17 | ||
| − | |||
| − | |||
| − | |||
| − | |||
| + | Concentrations above 0,5 mg/l can cause acute [[toxic|toxicity]] in sandworms<ref name = ken>Kennish, M. J. (1996): Practical Handbook of Estuarine and Marine Pollution, CRC Press 524 pp</ref>. | ||
| + | Concentrations in sediments of highly polluted areas can reach up to 400 µg/kg in the sediments<ref name = ken>Kennish, M. J. (1996): Practical Handbook of Estuarine and Marine Pollution, CRC Press 524 pp</ref>. | ||
<P> | <P> | ||
<BR> | <BR> | ||
<P> | <P> | ||
| + | |||
== Environmental standards and legislation == | == Environmental standards and legislation == | ||
| Line 44: | Line 42: | ||
==References== | ==References== | ||
<references/> | <references/> | ||
| + | {{author | ||
| + | |AuthorID=19826 | ||
| + | |AuthorFullName=Daphnis De Pooter | ||
| + | |AuthorName=Daphnisd}} | ||
| − | [[Category: | + | [[Category:Toxicity chemicals]] |
Latest revision as of 13:14, 9 August 2020
Definition of fluoranthene:
Fluoranthene is a polycyclic aromatic hydrocarbon (PAH). It exists as pale yellow needles or plates. Fluoranthene can be produced by the pyrolysis at high temperatures of organic raw materials such as coal and petroleum. It is also known to to be produced by certain plants. Fluoranthene is a constituent of coal tar and petroleum-derived asphalt. Currently, there is no known production or use of this compound[1].
This is the common definition for fluoranthene, other definitions can be discussed in the article
|
Notes
| Fluoranthene |
|---|
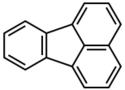
|
| Formula |
| C16H10 |
Since it is a universal product of combustion of organic matter and is present in fossil fuel products, fluoranthene is generally released into air and water. In the atmosphere fluoranthene is present both as vapour and adsorbed to particles. As vapour it will be degraded rather rapidly, but it's more stable when adsorbed to particles and as such fluoranthene can travel large distances before being deposited. Fluoranthene has a low water solubility of 265 µg/l, and will rapidly be adsorbed to sediment and particulate matter. It disappears from the water column, by degradation and transport to the sediments. In the sediments however, it can be very stable for decades. Since it has a high tendency to adsorb to organic matter, it has a high potential towards bioaccumulation. It bioaccumulates in shellfish, making them an excellent bioindicator for fluoranthene pollution[2].
Concentrations above 0,5 mg/l can cause acute toxicity in sandworms[3].
Concentrations in sediments of highly polluted areas can reach up to 400 µg/kg in the sediments[3].
Environmental standards and legislation
Included in the water framework list of priority substances
See also
Fluoranthene on the ED North Database
References
- ↑ The Risk Assessment Information System Toxicity Summary for FLUORANTHENE August 17 2009
- ↑ www.speclab.com August 17 2009
- ↑ 3.0 3.1 Kennish, M. J. (1996): Practical Handbook of Estuarine and Marine Pollution, CRC Press 524 pp
Please note that others may also have edited the contents of this article.
|