Difference between revisions of "Coastal pollution and impacts"
Dronkers J (talk | contribs) |
|||
| (30 intermediate revisions by 6 users not shown) | |||
| Line 1: | Line 1: | ||
| − | |||
| − | |||
| + | Coastal and estuarine [[ecosystem]]s have been, and still are, heavily influenced by humans through pollution and habitat loss worldwide. Over 80% of all marine [[pollution]] originates from land-based sources which are primarily industrial, agricultural and urban <ref>Cicin-Sain, B., Balgos, M., Appiott, J., Wowk, K. and Hamon, G. 2012. Oceans at Rio+20: How Well Are We Doing in Meeting the Commitments from the 1992 Earth Summit and the 2002 World Summit on Sustainable Development? Summary for Decision Makers. University of Delaware and Global Ocean Forum.</ref>. Pollution accompanies most kinds of human activities, including offshore oil and gas production and marine oil transportation. Besides altering the marine environment, pollution also causes economic losses<ref>Ofiara D. and Seneca, J. 2006. Biological effects and subsequent economic effects and losses from marine pollution and degradations in marine environments: Implications from the literature. Marine Pollution Bulletin 52: 844-864.</ref>. | ||
| − | |||
==Background== | ==Background== | ||
| − | The relative contribution of each | + | The relative contribution of each human activity to the overall pollution impact depends on the specific situation and on the released substances. Quantitative estimates are difficult because of the lack of reliable data and the extreme complexity of biogeochemical cycles, especially at the sea-land and sea-atmosphere interfaces. |
| − | Point and non-point source | + | Point and non-point source pollutions continue globally, resulting in the steady degradation of coastal and marine [[ecosystem]]s. Indirect (or diffuse) inputs are usually widespread low-level discharges and likely result in chronic pollution. In this section, generic sources of pollution and effects ([[eutrophication]], contamination and pollution from industry and agriculture, etc.) are considered. The sensitivity of the [[coastal zone]] to watershed impacts is examined in relation to land-derived pollution and water quality. |
| − | [[Contamination]] is usually considered | + | [[Contamination]] is usually considered a lesser degree of pollution, that occurs when an input of waste from human activities increases the concentration of a substance in seawater, sediments or an animal above the background level for that area or animal, but without (immediate) obvious effect. |
| + | |||
==Agriculture== | ==Agriculture== | ||
| − | Emissions and inputs from agriculture are a significant source of | + | Emissions and inputs from agriculture are a significant source of pollution to the [[coastal zone]] and to the atmosphere throughout the world. The contribution of agriculture to the pollution of coastal [[ecosystem]]s affects biogeochemical cycles, notably in terms of <ref> Mateo-Sagasta, J., Zadeh, S.M. and Turral, H. 2017. Water pollution from agriculture: a global review. Executive summary. FAO (UN) and International Water Management Institute. </ref>: |
*Discharge of methane and ammonia contributing to the greenhouse effect | *Discharge of methane and ammonia contributing to the greenhouse effect | ||
*Use of insecticides and other pesticides affecting species, cultivated or not | *Use of insecticides and other pesticides affecting species, cultivated or not | ||
| − | *[[Pollution]], from use of oil for instance | + | *[[Pollution]], from use of oil, for instance |
| − | *[[ | + | *[[Nutrient]]s run-off from silage and slurry-manure, use of fertilizers leading to [[eutrophication]] |
===Eutrophication=== | ===Eutrophication=== | ||
| − | [[ | + | |
| − | [[ | + | [[File:EutrophicationZostera.jpg|thumb|300px|right|Eutrophication: Overgrown Zostera in the Baltic Sea. Photo credit Metsähallitus. Creative Commons Licence]] |
| − | In the recent past [[eutrophication]] has been most pronounced in the developed world, but it has to be expected that it will become more and more important in the developing countries of Asia, Africa and Latin America in the near future<ref>Nixon, 1995</ref>. | + | |
| + | [[Eutrophication]] results from the increase of nutritional resources to a particular water body and includes the supply of mineral [[nutrient]]s (nitrogen, phosphorus, silicon, trace elements) as well as organic carbon<ref> Richardson, K., Jorgensen, B.B., 1996. Eutrophication: definition, history and effects. Eutrophication in Coastal Marine Ecosystems. Coastal and Estuarine Studies, vol. 52. American Geophysical Union, pp. 1–19.</ref>. Discharges and emissions from land-based sources (industry, households, traffic, agriculture) provide large inputs of [[nutrient]]s to coastal waters via rivers, direct discharges, diffuse sources and deposition from the atmosphere. However, [[eutrophication]] cannot be defined just in terms of an increase in [[nutrient]]s concentration, as its manifestations (very often harmful to [[ecosystem]]s) occur due to the existence of natural conditions, such as high temperatures and calm coastal waters. | ||
| + | In the recent past [[eutrophication]] has been most pronounced in the developed world, but it has to be expected that it will become more and more important in the developing countries of Asia, Africa and Latin America in the near future<ref>Nixon, S.W. 1995. Coastal marine eutrophication: A definition, social causes, and future concerns. Ophelia 41: 199-219.</ref>. | ||
Additional atmospheric input of inorganic [[nitrogen]] has increased significantly to a level where it is already higher than the natural [[nitrogen]] supply in the North Atlantic Ocean basin. It is expected that the worldwide production of [[nitrogen]] (mainly from fertilizer industry and the burning of fossil fuel) will affect the biogeochemical cycles on a global scale. Unfortunately, the interactive effects of the altering N-cycle on the carbon cycle (including the dynamics of greenhouse gases within both cycles) are poorly understood. | Additional atmospheric input of inorganic [[nitrogen]] has increased significantly to a level where it is already higher than the natural [[nitrogen]] supply in the North Atlantic Ocean basin. It is expected that the worldwide production of [[nitrogen]] (mainly from fertilizer industry and the burning of fossil fuel) will affect the biogeochemical cycles on a global scale. Unfortunately, the interactive effects of the altering N-cycle on the carbon cycle (including the dynamics of greenhouse gases within both cycles) are poorly understood. | ||
| − | The effects of [[eutrophication]] vary from increased growth of [[phytoplankton]], benthos and fish to changed species composition at moderate [[eutrophication]]. | + | The effects of [[eutrophication]] vary from increased growth of [[phytoplankton]], benthos and fish to changed species composition at moderate [[eutrophication]]. At severe [[eutrophication]], the effects vary from blooms of nuisance-causing or toxic algae to mass growth of certain species and mortality of others, and ultimately to anoxic conditions and mass mortality (fishkills). An algal biomass related phenomenon such as oxygen depletion of the water column and consequent mortality of animals can be prevented by a general reduction of nutrient discharges. At present, mathematical models on [[ecosystem]] dynamics are reliable enough to estimate dose-effect relationships. Harmful effects related to algal species are less predictable. There is general consensus about a global increase in [[harmful algal bloom]]s. Also, there are reports which suggest a link between blooms of toxic [[algae]] and human activities such as salmon (or fish) farming <ref> |
| + | Anderson, D.M., Burkholder, J.M., Cochlan, W.P., Glibert, P.M., Gobler, C.J., Heil, C.A., Kudela, R., Parsons, M.L., Rensel, J. E., Townsend, D.W., Trainer, V.L. and Vargol, G.A. 2008. Examining linkages from selected coastal regions of the United States. Harmful Algae 8: 39-53.</ref> Changes in N:P:Si ratios may also cause a shift in species composition. Consequently, alterations in [[pelagic]] and [[benthic]] communities are to be expected. Which species is stimulated by [[eutrophication]] is highly dependent on the local environmental conditions <ref>Anderson, D.M., Cembella, A.D. and Hallegraeff, G.M. (editors) 1998. Physiological Ecology of Harmful Algal Blooms. Springer.</ref>. | ||
| + | |||
| + | See also: | ||
| + | :[[What causes eutrophication?]] | ||
| + | :[[Possible consequences of eutrophication]] | ||
| + | :[[Nutrient conversion in the marine environment]] | ||
| + | :[[Harmful algal bloom]] | ||
| + | :other articles in the category [[:Category:Eutrophication|Eutrophication]]. | ||
==Industry== | ==Industry== | ||
| − | Since most | + | Since most contaminants enter the sea by flows from the surrounding land, in particular via rivers, the highest concentrations are often found in estuaries and coastal areas and thus maximal effects of contaminants on the [[ecosystem]] could be expected to occur here. This general picture can be influenced by additional inputs from sources at sea - ships, off-shore platforms - and by inputs via the atmosphere. After entering the sea, contaminants are usually diluted and widely dispersed. However, the adsorption of contaminants to suspended solid material in the sea leads to the occurrence of elevated concentrations in the seabed in areas where this material settles. Areas, which are also close to direct sources of input, are particularly at risk, for example estuaries and lagoons. |
| − | Water quality is affected by toxic substances that are persistent in the marine environment. These substances are of varying origin and composition, but they are together | + | |
| + | Water quality is affected by toxic substances that are persistent in the marine environment. These substances are of varying origin and composition, but they are together classified as stable or persistent, and have decisive properties in common. They are not readily degradable, or not at all degradable, toxic to living organisms, and bio-available (living organisms can take them up and accumulate them). The persistence of certain groups of contaminants, recognised as “toxic” in the marine environment, varies. Some of the factors that have an influence include their chemical reactivity, which describes the probability for reactions with other substances; photochemical reactivity is an example that deals with the probability of reactions initiated by light. Their biological reactivity is dependent upon the probability for different biological systems to modify or metabolise a chemical compound. In turn, the distribution and availability of chemicals in the environment depend on a number of parameters, such as: | ||
*''The emission'' - whether the contaminant is emitted as a gas, a solution, adsorbed on particles or included in a solid matrix, and | *''The emission'' - whether the contaminant is emitted as a gas, a solution, adsorbed on particles or included in a solid matrix, and | ||
| − | *''The | + | *''The volatility'' - that influences the distribution of the chemical; |
*''The lipophilicity'' - depends on the solubility of the chemical in lipids and is one of the factors affecting accumulation in organisms; | *''The lipophilicity'' - depends on the solubility of the chemical in lipids and is one of the factors affecting accumulation in organisms; | ||
*''The structure of the molecule'' - influences the stability and the biological availability of the chemical. | *''The structure of the molecule'' - influences the stability and the biological availability of the chemical. | ||
| − | *''The geographical distribution'' of | + | *''The geographical distribution'' of pollutants - is dependent on partition between different compartments. The following factors can have a significant influence: |
*''The source pattern'' - whether it is a point or a diffuse source, | *''The source pattern'' - whether it is a point or a diffuse source, | ||
*''The wind'' - is essential for a long range transport, | *''The wind'' - is essential for a long range transport, | ||
*''The precipitations'' - are an important mode of transport from the air to seawater and to sediments, | *''The precipitations'' - are an important mode of transport from the air to seawater and to sediments, | ||
| − | *''The contribution from the watershed and riverine'' inputs - are | + | *''The contribution from the watershed and riverine'' inputs - are dependent upon factors such as the geology, the geomorphology, the relief… |
| − | *''The currents'' in the sea are | + | *''The currents'' in the sea are dependent on tides, location, exposure... |
*''The movements of organisms'' - are another transport mode of contaminants. | *''The movements of organisms'' - are another transport mode of contaminants. | ||
| − | '''''Bio-concentration''''' or '''''[[ | + | The environmental impact of persistent chemical pollutants depends to a large degree on '''''Bio-concentration''''' or '''''[[Bioaccumulation]]''''' of contaminants in the tissues of organisms. [[Bioaccumulation]] describes the ratio of a chemical in the organism and the concentration in the surrounding medium. This factor is a function of the stability of the chemical but also how it will accumulate in the fat of the body, i.e. its lipophilicity. Accumulation presents a risk to consumer organisms, including the human species. |
| − | '''''[[ | + | '''''[[Biomagnification]]''''' describes a higher concentration in an organism than in its prey. It is related to the biological availability of contaminants to organisms and to their metabolism and excretion rate. Therefore, identical levels of a specific contaminant can have different effects due to the fact that they can be present in different forms with different availability for uptake. |
| − | ===Organic | + | ===Persistent Organic Pollutants (POPs)=== |
| − | More than 7,000,000 | + | More than 7,000,000 synthetic organic substances are known as Persistent Organic Pollutants (POPs) and there are almost infinite possibilities to combine new substances. Serious environmental damage is caused by some of these POPs in the sea<ref> Takada, H. and Yamashita, R. 2016. Chapter 7.2: Pollution status of Persistent Organic Pollutants. In: Large Marine Ecosystems: Status and trends. UNEP, Nairobi, pp. 165-176.</ref>. Effects take place at metabolic and physiological levels, both in marine vertebrates and invertebrates. The great number of organic substances are due to the numerous possible substituents and substitution patterns. In fact, the acute toxicity of compounds is only relevant after accidental spills. The only important exception to this rule is the bird casualties due to operational spills from ships of lipophilic floating substances and surfactants. The more important effects to focus on from a scientific point of view are caused by chronic exposure to relatively low concentrations and affect reproduction, immunology and carcinogenicity. |
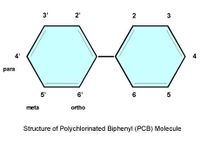
| − | + | [[File:PCB.jpg|thumb|200px|right|Molecular structure polychlorinated biphenyl (PCB)]] | |
| − | Organometallic compounds such as tributyltin have been used extensively as antifouling agents and are now banned in many countries because of its effect known as ''imposex''. Imposex refers to a change of sexual characteristics in invertebrates, female gastropods growing a penis, for instance. Compounds in alternative antifouling products | + | In common, POPs all have the following characteristics: they are stable and toxic, and share a similar structure. It has emerged that some highly stable organic compounds - chiefly halogenated hydrocarbons - can have serious environmental effects in the sea. Such substances have in common the presence of a halogen in their molecule (chlorine, iodine, fluorine, astatine), have a low polarity and low water solubility. Aromatic compounds are more reactive and susceptible to chemical and biochemical transformation and include pesticides (chlorinated such as [[DDT]] – DDE, [[PAH|Polycyclic Aromatic Hydrocarbons]], Hexa Cyclo Hexan, and organometallics such as [[TBT|tributyltin]]). There are 209 congeners of Poly Chloro Biphenyls, all with different properties. This variety makes both analysis and effect studies complicated. The metabolic pathways of [[PCB|polychlorinated biphenyls]] (PCBs) congeners are complex. Though the use of PCBs has been prohibited for a long time, emissions from unidentified sites still occur. For example, in the recycling of materials to which toxic chemicals have been added<ref>Pivnenko, K., Eriksson, E. and Astrup, T. 2015. Waste paper for recycling: Overview and identification of potentially critical substances. Waste Manag. 45:134-42. doi: 10.1016/j.wasman.2015.02.028.</ref>. It is unclear how organic synthetic organic chemicals affect marine organisms but PCBs, for instance, are frequently found in fish liver, seal blubber, bird eggs, and human fat. [[Organochlorine compounds|Organochlorines]] have been associated with impaired reproductive ability in [[pollution and marine mammals|seals and whales]]. For instance, octachlorostyrene (OCSs) have been found in [[benthic]] organisms. OCS concentrations can be taken as an indication of incomplete combustion resulting in the accumulation of chlorinated hydrocarbons in marine organisms. |
| + | |||
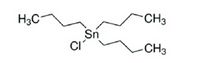
| + | [[File:Tributyltin.jpg|thumb|right|200px|Molecular structure tributyltin (TBT)]] | ||
| + | |||
| + | Organometallic compounds such as tributyltin have been used extensively as [[antifouling agent|antifouling agents]] and are now banned in many countries because of its effect known as [[TBT and Imposex|''imposex'']]. Imposex refers to a change of sexual characteristics in invertebrates, female gastropods growing a penis, for instance. Compounds in alternative antifouling products require serious scrutiny to ensure that these compounds do not cause toxic effects in the marine environment. Their use is expected to increase in the near future due to the total ban on tributyltin-based antifouling chemicals by the ''International Maritime Organisation'' [http://www.imo.org/ IMO] in 2003. In well-oxygenated surficial sediments, TBT typically | ||
| + | has a half-life of 1–5 years, but in fine-grained, O<sub>2</sub>-depleted - anoxic sediments the half-life can extend to several decades. Since recovery from contamination is slow, TBT hotspots are still found around major ports and shipyards worldwide. <ref>Beyer, J., Song, Y., Tollefsen, K.E., Berge, J.A., Tveiten, L., Helland, A., Oxnevad, S. and Schoyen, M. 2022. The ecotoxicology of marine tributyltin (TBT) hotspots: A review. Marine Environmental Research 179, 105689</ref> | ||
| + | |||
| + | See also: | ||
| + | :[[Plastics in the ocean]] | ||
| + | :[[Pollution prevention, detection and mitigation in European coastal waters]] | ||
| + | :[[Antifouling paints]] | ||
| + | :[[Portal: Ecotox]] | ||
====Effect on wildlife==== | ====Effect on wildlife==== | ||
| − | It is now well established that there are [[anthropogenic]] chemicals released to the environment that can disrupt the endocrine systems of a wide range of wildlife species. The reproductive hormone-receptor systems appear to be especially vulnerable. Indeed, changes in sperm counts, genital tract malformations, infertility, an increased frequency of mammary, prostate and testicular tumours, feminisation of male individuals of diverse vertebrate species and altered reproductive behaviours, have all been reported | + | It is now well established that there are [[anthropogenic]] chemicals released to the environment that can disrupt the endocrine systems of a wide range of wildlife species. The reproductive hormone-receptor systems appear to be especially vulnerable. Indeed, changes in sperm counts, genital tract malformations, infertility, an increased frequency of mammary, prostate and testicular tumours, feminisation of male individuals of diverse vertebrate species and altered reproductive behaviours, have all been reported <ref>Sharpe, R.M. and Skakkebaek, N.E. 1993. Are oestrogens involved in falling sperm counts and disorders of the male reproductive tract? Lancet 341, 1392–1395.</ref><ref>Colborn, T. 1995. Pesticides—how research has succeeded and failed to translate science into policy: endocrinological effects on wildlife. Environ Health Perspect 103: 81–85.</ref>. With regard to environmental management, the problem of [[endocrine disrupting compounds|endocrine disrupting chemicals]] is extremely difficult to address. Basic research is required to strengthen the scientific foundation for risk assessment: e.g., baseline studies on endocrine dysfunction across classes of animals to reduce the uncertainty associated with species extrapolations <ref>NSTC 1996. The health and ecological effects of endocrine disrupting chemicals. A framework for planning. Committee on Environment and Natural Resources, National Science and Technology Council (USA).</ref>. See also articles under the [[Portal:Ecotox|Ecotox Portal]]. |
| − | An extensive list of chemicals which are thought to be capable of disrupting the reproductive endocrine systems of animals has been assembled. They fall into the following categories: | + | An extensive list of chemicals which are thought to be capable of disrupting the reproductive endocrine systems of animals has been assembled<ref> Thomas K.V. and Hilton M.J. 2004. The occurrence of selected human pharmaceutical compounds in UK estuaries. Marine Pollution Bulletin 49: 436-444.</ref>. They fall into the following categories: |
| − | #Environmental oestrogens (oestrogen receptor mediated) (e.g.,'' methoxychlor, | + | #Environmental oestrogens (oestrogen receptor mediated) (e.g.,'' [[methoxychlor]], bisphenol compounds)''; |
| − | #Environmental antioestrogens (e.g., ''Dioxin, | + | #Environmental antioestrogens (e.g., ''[[Dioxins|Dioxin]], [[Endosulfan]]''); |
#Environmental antiandrogens (e.g., ''Vinclozolin, DDE, Kraft mill effluent''); | #Environmental antiandrogens (e.g., ''Vinclozolin, DDE, Kraft mill effluent''); | ||
| − | #Toxicants that reduce steroid hormone levels (e.g., ''Fenarimol and other fungicides; | + | #Toxicants that reduce steroid hormone levels (e.g., ''Fenarimol and other fungicides; [[endosulfan]]''); |
#Toxicants that affect reproduction primarily through effects on the CNS (e.g., ''dithiocarbamate pesticides, methanol''); and | #Toxicants that affect reproduction primarily through effects on the CNS (e.g., ''dithiocarbamate pesticides, methanol''); and | ||
| − | #Other toxicants that affect hormonal status (e.g., ''cadmium, benzidine-based dyes''). | + | #Other toxicants that affect hormonal status (e.g., ''[[cadmium]], benzidine-based dyes''). |
| + | |||
| + | See also: | ||
| + | :[[Endocrine disrupting compounds in the coastal environment]] | ||
===Metals=== | ===Metals=== | ||
| − | Heavy metals are naturally occurring and do not degrade. They are not particularly toxic as | + | [[Heavy metals]] are naturally occurring and do not degrade. They are not particularly toxic as condensed free elements (except [[Mercury]] (Hg) vapour) but they are dangerous to living organisms in the form of cations and when bonded to short chains of carbon atoms. Cations In particular have a strong affinity for sulphur. For example, sulfhydril groups in enzymes attach to cations or molecules and so the enzyme is blocked. They are a problem in the marine environment because they [[bioaccumulation|bioaccumulate]] in marine organisms and despite measures taken to combat [[pollution]], the concentration still increases. [[Pollution]] above background levels in the environment can cause serious effects. For instance, [[copper]] is a useful oligoelement but in excess it affects [[Trophic level - a marine example|trophic levels]]. As a free element, [[Mercury pollution|mercury]] has hundreds of applications, for example in electrical switches. Despite emissions of vapour from the industry have been curtailed, there are still releases from unregulated burning of fuel or wastes. Human sources of pollutants add up in the atmospheric; the total contribution is of the same order of magnitude as the contribution of volcanoes. The ultimate sink for metals and many organic compounds is the sediment. Processes contributing to deposition and burial of heavy metals are: |
*adsorption onto the surface of mineral particles; | *adsorption onto the surface of mineral particles; | ||
*complexation by molecules in organic particles, and | *complexation by molecules in organic particles, and | ||
| Line 71: | Line 95: | ||
===Radioactive substances=== | ===Radioactive substances=== | ||
| − | Present day levels of | + | Present day levels of radioactive substances found in coastal waters are the result of natural radioactivity (cosmic rays, earth's crust), and radioactivity released due to human activities such as oil exploration and combustion, phosphate production and use, land-based mining, medical diagnosis and therapy and food conservation. The world's oceans have been a sink for radioactive waste from the production of nuclear weapons and from electric power and reprocessing facilities since 1944. Radioactive waste enters the ocean from nuclear weapon testing and the resulting atmospheric fallout, the releasing or dumping of wastes from nuclear fuel cycle systems, and nuclear accidents (for example Chernobyl in 1985). Dumping of high-level radioactive waste is no longer permitted in the ocean but dumping of low-level wastes is still permitted. Low-level waste contains less radioactivity per gram than high-level waste. High-level wastes usually have longer half-lives. For example, one common high-level waste that is produced by spent nuclear fuel has a half-life of 24,100 years. |
| − | The world's oceans have been a sink for radioactive waste from the production of nuclear weapons and electric power since 1944. Radioactive waste enters the ocean from nuclear weapon testing and the resulting atmospheric fallout, the releasing or dumping of wastes from nuclear fuel cycle systems, and nuclear accidents (for example Chernobyl in 1985). Dumping of high-level radioactive waste is no longer permitted in the ocean | ||
===Oil and gas and offshore installations=== | ===Oil and gas and offshore installations=== | ||
| − | Oil is at the heart of the modern economy in providing a cheap source of energy and as a raw material for making plastics, etc. It is a mixture of | + | Oil is at the heart of the modern economy in providing a cheap source of energy and as a raw material for making plastics, etc. It is a mixture of hydrocarbons and up to 25% non-hydrocarbons such as sulphur, vanadium, and metals. Environmental impacts occur at all stages of oil and gas production and use. They result from prospecting activities (including seismic techniques), physical impact due to the installation of rigs, operational discharges when production starts, accidental and routine spills, and finally combustion. Nihoul and Ducrotoy (1994)<ref>Nihoul, C. and Ducrotoy, J-P. 1994. Impact of oil on the marine environment: Policy of the Paris commission on operational discharges from the offshore industry. Marine Pollution Bulletin 29: 323-329.</ref> have estimated the input of oil to the North Sea, due to the offshore industry, at 29% of the total input of oil. Offshore installations may disturb the environment through the placement of structures on the seabed, producing underwater noise and light emissions and disturbing [[benthic]] organisms. An increasing number of installations are currently reaching the end of their productive life and will need to be dismantled or removed throughout the worlds seas. |
| + | |||
| + | Overall, coastal [[ecosystem]]s remain largely affected by direct discharges of oil from offshore activities and illegal discharges from ships. Operational discharges consist of production water and drilling cuttings. Although the amount of oil discharged via production water is increasing as platforms are getting older, cuttings still account for 75% of the oil entering the sea as a result of normal operations. The effects on the marine environment have been extensively studied by national authorities as well as by the industry. See also [[Oil spill pollution impact and recovery]], [[Oil spill monitoring]] and [[Index of vulnerability of littorals to oil pollution]]. | ||
| + | |||
| + | Despite uncertainty about possible long-term effects, problems with oiled cuttings (drilling muds) are acknowledged. Effects in the vicinity of platforms are well known, in particular those on the macrobenthic invertebrates, which range from lethal to insignificant, depending on proximity<ref> Grant A. and Briggs A.D. 2002. Toxicity of sediments from around a North Sea oil platform: are metals or hydrocarbons responsible for ecological impacts? Marine Environmental Research 53: 95-116.</ref>. Spills of oil and the release of chemicals (i.e. lubricants) used during exploitation constitute an important source of pollution to coastal seas. The experience gained from several major oil spill accidents is summarized in the article [[Oil spill pollution impact and recovery]]. | ||
| + | |||
| + | [[Image:oiled bird.jpg|thumb|Oil spill pollution: Oil-smeared bird. Photo credit USGS.]] | ||
| + | |||
| + | Even if accidental spills represent a relatively small source of oil, they directly affect birds and mammals and have devastative effects on local vulnerable economies (see [[Index of vulnerability of littorals to oil pollution]] and [[Overview of oil spills events from 1970 to 2000]]). Shipping is a main source of oil slicks (chronic and accidental) showing no downward trend (see [[North Sea pollution from shipping: legal framework]]). Combustion of oil is the ultimate stage in the chain of production-use. Polycyclic aromatic hydrocarbons (PAHs) originate from various sources such as flaring or engines, including on land. Nevertheless, their major sources are not the rivers but sources in the sea itself from platforms and ships <ref>Laane, R.W.P.M., Sonneveldt, H.L.A., Van der Weyden, A.J., Loch, J.P.G. and Groeneveld, G. 1999. Trends in the spatial and temporal distribution of metals (Cd, Cu, Zn and Pb) and organic compounds (PCBs and PAHs) in Dutch coastal zone sediments from 1981 to 1996: a model case study for Cd and PCBs. Journal of Sea Research 41: 1–17.</ref> . PAHs are volatile material which travel well airborne, showing the importance of the atmospheric pathway in the distribution of contaminants. Airborne pollutants are dissolved by rain and some are carried to the coast as dust particles or in solution from the atmosphere. The current knowledge about the dependence of the deposition velocity upon the particle size and about the processes controlling wet deposition fluxes, and the quality and completeness of the emission data are still inadequate for describing the environmental cycle and impact of such pollutants. | ||
| + | |||
| + | ===Sound pollution=== | ||
| + | Another type of pollution is underwater noise, sound pollution produced by human activities such as trawling, dredging, military exercises, oil and gas exploration, seismic surveys, commercial shipping, recreational boats, windfarm construction (e.g. pile driving), sounds emitted by turbines, etc. Underwater noise has serious detrimental effects on many marine animals, described in the article [[Underwater noise]]. | ||
| − | |||
| − | + | ==Brine discharge== | |
| − | + | The growing demand for fresh water in arid countries has led to a tremendous increase in the number of desalination plants and a corresponding increase in desalination capacity over the past decennia. More than 80% of the desalination plants are located in the coastal zone; they are fed with seawater and discharge the effluent (called brine) into the coastal ocean. In 2019, global brine discharge was estimated at about 125 million m3/day. <ref>Jones, E., Qadir, M., van Vliet, M.T.H., Smakhtin, V. and Kang, S-M. 2019. The state of desalination and brine production: A global outlook. Science of the Total Environment 657: 1343–1356</ref> The brine disposal, which spreads along the seabed, has a strong impact on the benthic coastal ecosystem, not only due the high salinity of around 60 CPU, but also due to increased turbidity and decreased oxygen levels in the brine layer. Water quality is affected by toxic chemicals (e.g. chlorine, polyphosphates, sulfuric acid and traces of copper, iron, nickel, chromium, molybdenum) used in water pretreatment or as anti-scalants and anti-foulants in the desalination process. Seagrass and coral reef habitats are severely affected by brine disposal.<ref>Roberts, D.A., Johnston, E.L. and Knott, N.A. 2010. Impacts of desalination plant discharges on the marine environment: a critical review of published studies. Water Res. 44: 5117–5128</ref> | |
==Conclusion== | ==Conclusion== | ||
| − | The effects of contaminants on coastal [[ | + | The effects of contaminants on coastal [[ecosystem]]s are very difficult to assess. In the estimation of possible effects, the actual concentrations are compared with the levels that can cause effects. Results of laboratory experiments give only limited information in relation to the field situation, due to the complexity of natural systems and (in general) the co-occurrence of a multitude of contaminants in the field. Actual changes in the sea are often very difficult to discern from the large variability, which occurs naturally. |
| + | |||
| + | Measurement of marine pollution using [[bioindicator|biological indicators]] provides information on the bio-availability of contaminants and the integration of the effects of multiple exposures and exposure over time. Multiple exposure is the combined action of all chemical contaminants. Even if the contaminants are present at concentrations too low to cause gross harmful effects, they can cause a suite of biochemical reactions in marine organisms generally called stress. Amongst the result of prolonged stress is the suppression of the immune system, thus increasing sensitivity towards the impact of infectious agents and parasites. | ||
| + | |||
| + | Natural factors such as temperature extremes and fluctuations of salinity or [[anthropogenic]] activities, such as [[Effects_of_fisheries_on_marine_biodiversity|fisheries]], can aggravate these reactions. A suite of biochemical reactions in marine organisms may occur as a response of increasing sensitivity towards the impact of infectious agents and parasites. | ||
| − | |||
| − | |||
| − | == | + | ==Related articles== |
| − | + | :[[Threats to the coastal zone]] | |
| + | :[[Environmental risk assessment of marine activities]] | ||
| + | For an overview of specific polluting substances and for information on their properties and effects see articles under the category [[:Category:Coastal and marine pollution|Coastal and marine pollution]]. | ||
| − | |||
| − | + | ==Further reading== | |
| − | + | Clark R. (2001). ''Marine pollution'', Oxford University Press. | |
Frankel E.B. (1995). ''Ocean Environmental Management'', Pearson Professional Education. | Frankel E.B. (1995). ''Ocean Environmental Management'', Pearson Professional Education. | ||
| Line 104: | Line 140: | ||
Garbuny Vogel C. (2003). ''Human Impact (Restless Sea)'', Franklin Watts. | Garbuny Vogel C. (2003). ''Human Impact (Restless Sea)'', Franklin Watts. | ||
| − | Gardes L. (2003). ''Endangered Oceans'', Greenhaven Press. | + | Gardes L.I. (2003). ''Endangered Oceans'', Greenhaven Press. |
Gorman M. (1993). ''Environmental Hazards: Marine Pollution'', ABC-CLIO, Santa Barbara, CA. | Gorman M. (1993). ''Environmental Hazards: Marine Pollution'', ABC-CLIO, Santa Barbara, CA. | ||
| − | + | Gren I.M., Turner and Wulff F. (2000). ''Managing a Sea'', Earthscan Publications Ltd, London. | |
| − | + | Talen M. (1991). ''Ocean Pollution'', Lucent Books,Gale Group, Farmington Hills, MI, U.S.A. | |
| − | |||
| − | |||
| − | + | ==References== | |
| + | <references/> | ||
| − | |||
| − | |||
| − | |||
{{author | {{author | ||
| Line 129: | Line 161: | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
[[Category:Coastal and marine pollution]] | [[Category:Coastal and marine pollution]] | ||
| − | |||
| − | |||
Latest revision as of 09:24, 1 October 2024
Coastal and estuarine ecosystems have been, and still are, heavily influenced by humans through pollution and habitat loss worldwide. Over 80% of all marine pollution originates from land-based sources which are primarily industrial, agricultural and urban [1]. Pollution accompanies most kinds of human activities, including offshore oil and gas production and marine oil transportation. Besides altering the marine environment, pollution also causes economic losses[2].
Contents
Background
The relative contribution of each human activity to the overall pollution impact depends on the specific situation and on the released substances. Quantitative estimates are difficult because of the lack of reliable data and the extreme complexity of biogeochemical cycles, especially at the sea-land and sea-atmosphere interfaces. Point and non-point source pollutions continue globally, resulting in the steady degradation of coastal and marine ecosystems. Indirect (or diffuse) inputs are usually widespread low-level discharges and likely result in chronic pollution. In this section, generic sources of pollution and effects (eutrophication, contamination and pollution from industry and agriculture, etc.) are considered. The sensitivity of the coastal zone to watershed impacts is examined in relation to land-derived pollution and water quality.
Contamination is usually considered a lesser degree of pollution, that occurs when an input of waste from human activities increases the concentration of a substance in seawater, sediments or an animal above the background level for that area or animal, but without (immediate) obvious effect.
Agriculture
Emissions and inputs from agriculture are a significant source of pollution to the coastal zone and to the atmosphere throughout the world. The contribution of agriculture to the pollution of coastal ecosystems affects biogeochemical cycles, notably in terms of [3]:
- Discharge of methane and ammonia contributing to the greenhouse effect
- Use of insecticides and other pesticides affecting species, cultivated or not
- Pollution, from use of oil, for instance
- Nutrients run-off from silage and slurry-manure, use of fertilizers leading to eutrophication
Eutrophication
Eutrophication results from the increase of nutritional resources to a particular water body and includes the supply of mineral nutrients (nitrogen, phosphorus, silicon, trace elements) as well as organic carbon[4]. Discharges and emissions from land-based sources (industry, households, traffic, agriculture) provide large inputs of nutrients to coastal waters via rivers, direct discharges, diffuse sources and deposition from the atmosphere. However, eutrophication cannot be defined just in terms of an increase in nutrients concentration, as its manifestations (very often harmful to ecosystems) occur due to the existence of natural conditions, such as high temperatures and calm coastal waters. In the recent past eutrophication has been most pronounced in the developed world, but it has to be expected that it will become more and more important in the developing countries of Asia, Africa and Latin America in the near future[5].
Additional atmospheric input of inorganic nitrogen has increased significantly to a level where it is already higher than the natural nitrogen supply in the North Atlantic Ocean basin. It is expected that the worldwide production of nitrogen (mainly from fertilizer industry and the burning of fossil fuel) will affect the biogeochemical cycles on a global scale. Unfortunately, the interactive effects of the altering N-cycle on the carbon cycle (including the dynamics of greenhouse gases within both cycles) are poorly understood.
The effects of eutrophication vary from increased growth of phytoplankton, benthos and fish to changed species composition at moderate eutrophication. At severe eutrophication, the effects vary from blooms of nuisance-causing or toxic algae to mass growth of certain species and mortality of others, and ultimately to anoxic conditions and mass mortality (fishkills). An algal biomass related phenomenon such as oxygen depletion of the water column and consequent mortality of animals can be prevented by a general reduction of nutrient discharges. At present, mathematical models on ecosystem dynamics are reliable enough to estimate dose-effect relationships. Harmful effects related to algal species are less predictable. There is general consensus about a global increase in harmful algal blooms. Also, there are reports which suggest a link between blooms of toxic algae and human activities such as salmon (or fish) farming [6] Changes in N:P:Si ratios may also cause a shift in species composition. Consequently, alterations in pelagic and benthic communities are to be expected. Which species is stimulated by eutrophication is highly dependent on the local environmental conditions [7].
See also:
- What causes eutrophication?
- Possible consequences of eutrophication
- Nutrient conversion in the marine environment
- Harmful algal bloom
- other articles in the category Eutrophication.
Industry
Since most contaminants enter the sea by flows from the surrounding land, in particular via rivers, the highest concentrations are often found in estuaries and coastal areas and thus maximal effects of contaminants on the ecosystem could be expected to occur here. This general picture can be influenced by additional inputs from sources at sea - ships, off-shore platforms - and by inputs via the atmosphere. After entering the sea, contaminants are usually diluted and widely dispersed. However, the adsorption of contaminants to suspended solid material in the sea leads to the occurrence of elevated concentrations in the seabed in areas where this material settles. Areas, which are also close to direct sources of input, are particularly at risk, for example estuaries and lagoons.
Water quality is affected by toxic substances that are persistent in the marine environment. These substances are of varying origin and composition, but they are together classified as stable or persistent, and have decisive properties in common. They are not readily degradable, or not at all degradable, toxic to living organisms, and bio-available (living organisms can take them up and accumulate them). The persistence of certain groups of contaminants, recognised as “toxic” in the marine environment, varies. Some of the factors that have an influence include their chemical reactivity, which describes the probability for reactions with other substances; photochemical reactivity is an example that deals with the probability of reactions initiated by light. Their biological reactivity is dependent upon the probability for different biological systems to modify or metabolise a chemical compound. In turn, the distribution and availability of chemicals in the environment depend on a number of parameters, such as:
- The emission - whether the contaminant is emitted as a gas, a solution, adsorbed on particles or included in a solid matrix, and
- The volatility - that influences the distribution of the chemical;
- The lipophilicity - depends on the solubility of the chemical in lipids and is one of the factors affecting accumulation in organisms;
- The structure of the molecule - influences the stability and the biological availability of the chemical.
- The geographical distribution of pollutants - is dependent on partition between different compartments. The following factors can have a significant influence:
- The source pattern - whether it is a point or a diffuse source,
- The wind - is essential for a long range transport,
- The precipitations - are an important mode of transport from the air to seawater and to sediments,
- The contribution from the watershed and riverine inputs - are dependent upon factors such as the geology, the geomorphology, the relief…
- The currents in the sea are dependent on tides, location, exposure...
- The movements of organisms - are another transport mode of contaminants.
The environmental impact of persistent chemical pollutants depends to a large degree on Bio-concentration or Bioaccumulation of contaminants in the tissues of organisms. Bioaccumulation describes the ratio of a chemical in the organism and the concentration in the surrounding medium. This factor is a function of the stability of the chemical but also how it will accumulate in the fat of the body, i.e. its lipophilicity. Accumulation presents a risk to consumer organisms, including the human species.
Biomagnification describes a higher concentration in an organism than in its prey. It is related to the biological availability of contaminants to organisms and to their metabolism and excretion rate. Therefore, identical levels of a specific contaminant can have different effects due to the fact that they can be present in different forms with different availability for uptake.
Persistent Organic Pollutants (POPs)
More than 7,000,000 synthetic organic substances are known as Persistent Organic Pollutants (POPs) and there are almost infinite possibilities to combine new substances. Serious environmental damage is caused by some of these POPs in the sea[8]. Effects take place at metabolic and physiological levels, both in marine vertebrates and invertebrates. The great number of organic substances are due to the numerous possible substituents and substitution patterns. In fact, the acute toxicity of compounds is only relevant after accidental spills. The only important exception to this rule is the bird casualties due to operational spills from ships of lipophilic floating substances and surfactants. The more important effects to focus on from a scientific point of view are caused by chronic exposure to relatively low concentrations and affect reproduction, immunology and carcinogenicity.
In common, POPs all have the following characteristics: they are stable and toxic, and share a similar structure. It has emerged that some highly stable organic compounds - chiefly halogenated hydrocarbons - can have serious environmental effects in the sea. Such substances have in common the presence of a halogen in their molecule (chlorine, iodine, fluorine, astatine), have a low polarity and low water solubility. Aromatic compounds are more reactive and susceptible to chemical and biochemical transformation and include pesticides (chlorinated such as DDT – DDE, Polycyclic Aromatic Hydrocarbons, Hexa Cyclo Hexan, and organometallics such as tributyltin). There are 209 congeners of Poly Chloro Biphenyls, all with different properties. This variety makes both analysis and effect studies complicated. The metabolic pathways of polychlorinated biphenyls (PCBs) congeners are complex. Though the use of PCBs has been prohibited for a long time, emissions from unidentified sites still occur. For example, in the recycling of materials to which toxic chemicals have been added[9]. It is unclear how organic synthetic organic chemicals affect marine organisms but PCBs, for instance, are frequently found in fish liver, seal blubber, bird eggs, and human fat. Organochlorines have been associated with impaired reproductive ability in seals and whales. For instance, octachlorostyrene (OCSs) have been found in benthic organisms. OCS concentrations can be taken as an indication of incomplete combustion resulting in the accumulation of chlorinated hydrocarbons in marine organisms.
Organometallic compounds such as tributyltin have been used extensively as antifouling agents and are now banned in many countries because of its effect known as imposex. Imposex refers to a change of sexual characteristics in invertebrates, female gastropods growing a penis, for instance. Compounds in alternative antifouling products require serious scrutiny to ensure that these compounds do not cause toxic effects in the marine environment. Their use is expected to increase in the near future due to the total ban on tributyltin-based antifouling chemicals by the International Maritime Organisation IMO in 2003. In well-oxygenated surficial sediments, TBT typically has a half-life of 1–5 years, but in fine-grained, O2-depleted - anoxic sediments the half-life can extend to several decades. Since recovery from contamination is slow, TBT hotspots are still found around major ports and shipyards worldwide. [10]
See also:
- Plastics in the ocean
- Pollution prevention, detection and mitigation in European coastal waters
- Antifouling paints
- Portal: Ecotox
Effect on wildlife
It is now well established that there are anthropogenic chemicals released to the environment that can disrupt the endocrine systems of a wide range of wildlife species. The reproductive hormone-receptor systems appear to be especially vulnerable. Indeed, changes in sperm counts, genital tract malformations, infertility, an increased frequency of mammary, prostate and testicular tumours, feminisation of male individuals of diverse vertebrate species and altered reproductive behaviours, have all been reported [11][12]. With regard to environmental management, the problem of endocrine disrupting chemicals is extremely difficult to address. Basic research is required to strengthen the scientific foundation for risk assessment: e.g., baseline studies on endocrine dysfunction across classes of animals to reduce the uncertainty associated with species extrapolations [13]. See also articles under the Ecotox Portal.
An extensive list of chemicals which are thought to be capable of disrupting the reproductive endocrine systems of animals has been assembled[14]. They fall into the following categories:
- Environmental oestrogens (oestrogen receptor mediated) (e.g., methoxychlor, bisphenol compounds);
- Environmental antioestrogens (e.g., Dioxin, Endosulfan);
- Environmental antiandrogens (e.g., Vinclozolin, DDE, Kraft mill effluent);
- Toxicants that reduce steroid hormone levels (e.g., Fenarimol and other fungicides; endosulfan);
- Toxicants that affect reproduction primarily through effects on the CNS (e.g., dithiocarbamate pesticides, methanol); and
- Other toxicants that affect hormonal status (e.g., cadmium, benzidine-based dyes).
See also:
Metals
Heavy metals are naturally occurring and do not degrade. They are not particularly toxic as condensed free elements (except Mercury (Hg) vapour) but they are dangerous to living organisms in the form of cations and when bonded to short chains of carbon atoms. Cations In particular have a strong affinity for sulphur. For example, sulfhydril groups in enzymes attach to cations or molecules and so the enzyme is blocked. They are a problem in the marine environment because they bioaccumulate in marine organisms and despite measures taken to combat pollution, the concentration still increases. Pollution above background levels in the environment can cause serious effects. For instance, copper is a useful oligoelement but in excess it affects trophic levels. As a free element, mercury has hundreds of applications, for example in electrical switches. Despite emissions of vapour from the industry have been curtailed, there are still releases from unregulated burning of fuel or wastes. Human sources of pollutants add up in the atmospheric; the total contribution is of the same order of magnitude as the contribution of volcanoes. The ultimate sink for metals and many organic compounds is the sediment. Processes contributing to deposition and burial of heavy metals are:
- adsorption onto the surface of mineral particles;
- complexation by molecules in organic particles, and
- precipitation reactions.
Radioactive substances
Present day levels of radioactive substances found in coastal waters are the result of natural radioactivity (cosmic rays, earth's crust), and radioactivity released due to human activities such as oil exploration and combustion, phosphate production and use, land-based mining, medical diagnosis and therapy and food conservation. The world's oceans have been a sink for radioactive waste from the production of nuclear weapons and from electric power and reprocessing facilities since 1944. Radioactive waste enters the ocean from nuclear weapon testing and the resulting atmospheric fallout, the releasing or dumping of wastes from nuclear fuel cycle systems, and nuclear accidents (for example Chernobyl in 1985). Dumping of high-level radioactive waste is no longer permitted in the ocean but dumping of low-level wastes is still permitted. Low-level waste contains less radioactivity per gram than high-level waste. High-level wastes usually have longer half-lives. For example, one common high-level waste that is produced by spent nuclear fuel has a half-life of 24,100 years.
Oil and gas and offshore installations
Oil is at the heart of the modern economy in providing a cheap source of energy and as a raw material for making plastics, etc. It is a mixture of hydrocarbons and up to 25% non-hydrocarbons such as sulphur, vanadium, and metals. Environmental impacts occur at all stages of oil and gas production and use. They result from prospecting activities (including seismic techniques), physical impact due to the installation of rigs, operational discharges when production starts, accidental and routine spills, and finally combustion. Nihoul and Ducrotoy (1994)[15] have estimated the input of oil to the North Sea, due to the offshore industry, at 29% of the total input of oil. Offshore installations may disturb the environment through the placement of structures on the seabed, producing underwater noise and light emissions and disturbing benthic organisms. An increasing number of installations are currently reaching the end of their productive life and will need to be dismantled or removed throughout the worlds seas.
Overall, coastal ecosystems remain largely affected by direct discharges of oil from offshore activities and illegal discharges from ships. Operational discharges consist of production water and drilling cuttings. Although the amount of oil discharged via production water is increasing as platforms are getting older, cuttings still account for 75% of the oil entering the sea as a result of normal operations. The effects on the marine environment have been extensively studied by national authorities as well as by the industry. See also Oil spill pollution impact and recovery, Oil spill monitoring and Index of vulnerability of littorals to oil pollution.
Despite uncertainty about possible long-term effects, problems with oiled cuttings (drilling muds) are acknowledged. Effects in the vicinity of platforms are well known, in particular those on the macrobenthic invertebrates, which range from lethal to insignificant, depending on proximity[16]. Spills of oil and the release of chemicals (i.e. lubricants) used during exploitation constitute an important source of pollution to coastal seas. The experience gained from several major oil spill accidents is summarized in the article Oil spill pollution impact and recovery.
Even if accidental spills represent a relatively small source of oil, they directly affect birds and mammals and have devastative effects on local vulnerable economies (see Index of vulnerability of littorals to oil pollution and Overview of oil spills events from 1970 to 2000). Shipping is a main source of oil slicks (chronic and accidental) showing no downward trend (see North Sea pollution from shipping: legal framework). Combustion of oil is the ultimate stage in the chain of production-use. Polycyclic aromatic hydrocarbons (PAHs) originate from various sources such as flaring or engines, including on land. Nevertheless, their major sources are not the rivers but sources in the sea itself from platforms and ships [17] . PAHs are volatile material which travel well airborne, showing the importance of the atmospheric pathway in the distribution of contaminants. Airborne pollutants are dissolved by rain and some are carried to the coast as dust particles or in solution from the atmosphere. The current knowledge about the dependence of the deposition velocity upon the particle size and about the processes controlling wet deposition fluxes, and the quality and completeness of the emission data are still inadequate for describing the environmental cycle and impact of such pollutants.
Sound pollution
Another type of pollution is underwater noise, sound pollution produced by human activities such as trawling, dredging, military exercises, oil and gas exploration, seismic surveys, commercial shipping, recreational boats, windfarm construction (e.g. pile driving), sounds emitted by turbines, etc. Underwater noise has serious detrimental effects on many marine animals, described in the article Underwater noise.
Brine discharge
The growing demand for fresh water in arid countries has led to a tremendous increase in the number of desalination plants and a corresponding increase in desalination capacity over the past decennia. More than 80% of the desalination plants are located in the coastal zone; they are fed with seawater and discharge the effluent (called brine) into the coastal ocean. In 2019, global brine discharge was estimated at about 125 million m3/day. [18] The brine disposal, which spreads along the seabed, has a strong impact on the benthic coastal ecosystem, not only due the high salinity of around 60 CPU, but also due to increased turbidity and decreased oxygen levels in the brine layer. Water quality is affected by toxic chemicals (e.g. chlorine, polyphosphates, sulfuric acid and traces of copper, iron, nickel, chromium, molybdenum) used in water pretreatment or as anti-scalants and anti-foulants in the desalination process. Seagrass and coral reef habitats are severely affected by brine disposal.[19]
Conclusion
The effects of contaminants on coastal ecosystems are very difficult to assess. In the estimation of possible effects, the actual concentrations are compared with the levels that can cause effects. Results of laboratory experiments give only limited information in relation to the field situation, due to the complexity of natural systems and (in general) the co-occurrence of a multitude of contaminants in the field. Actual changes in the sea are often very difficult to discern from the large variability, which occurs naturally.
Measurement of marine pollution using biological indicators provides information on the bio-availability of contaminants and the integration of the effects of multiple exposures and exposure over time. Multiple exposure is the combined action of all chemical contaminants. Even if the contaminants are present at concentrations too low to cause gross harmful effects, they can cause a suite of biochemical reactions in marine organisms generally called stress. Amongst the result of prolonged stress is the suppression of the immune system, thus increasing sensitivity towards the impact of infectious agents and parasites.
Natural factors such as temperature extremes and fluctuations of salinity or anthropogenic activities, such as fisheries, can aggravate these reactions. A suite of biochemical reactions in marine organisms may occur as a response of increasing sensitivity towards the impact of infectious agents and parasites.
Related articles
For an overview of specific polluting substances and for information on their properties and effects see articles under the category Coastal and marine pollution.
Further reading
Clark R. (2001). Marine pollution, Oxford University Press.
Frankel E.B. (1995). Ocean Environmental Management, Pearson Professional Education.
Garbuny Vogel C. (2003). Human Impact (Restless Sea), Franklin Watts.
Gardes L.I. (2003). Endangered Oceans, Greenhaven Press.
Gorman M. (1993). Environmental Hazards: Marine Pollution, ABC-CLIO, Santa Barbara, CA.
Gren I.M., Turner and Wulff F. (2000). Managing a Sea, Earthscan Publications Ltd, London.
Talen M. (1991). Ocean Pollution, Lucent Books,Gale Group, Farmington Hills, MI, U.S.A.
References
- ↑ Cicin-Sain, B., Balgos, M., Appiott, J., Wowk, K. and Hamon, G. 2012. Oceans at Rio+20: How Well Are We Doing in Meeting the Commitments from the 1992 Earth Summit and the 2002 World Summit on Sustainable Development? Summary for Decision Makers. University of Delaware and Global Ocean Forum.
- ↑ Ofiara D. and Seneca, J. 2006. Biological effects and subsequent economic effects and losses from marine pollution and degradations in marine environments: Implications from the literature. Marine Pollution Bulletin 52: 844-864.
- ↑ Mateo-Sagasta, J., Zadeh, S.M. and Turral, H. 2017. Water pollution from agriculture: a global review. Executive summary. FAO (UN) and International Water Management Institute.
- ↑ Richardson, K., Jorgensen, B.B., 1996. Eutrophication: definition, history and effects. Eutrophication in Coastal Marine Ecosystems. Coastal and Estuarine Studies, vol. 52. American Geophysical Union, pp. 1–19.
- ↑ Nixon, S.W. 1995. Coastal marine eutrophication: A definition, social causes, and future concerns. Ophelia 41: 199-219.
- ↑ Anderson, D.M., Burkholder, J.M., Cochlan, W.P., Glibert, P.M., Gobler, C.J., Heil, C.A., Kudela, R., Parsons, M.L., Rensel, J. E., Townsend, D.W., Trainer, V.L. and Vargol, G.A. 2008. Examining linkages from selected coastal regions of the United States. Harmful Algae 8: 39-53.
- ↑ Anderson, D.M., Cembella, A.D. and Hallegraeff, G.M. (editors) 1998. Physiological Ecology of Harmful Algal Blooms. Springer.
- ↑ Takada, H. and Yamashita, R. 2016. Chapter 7.2: Pollution status of Persistent Organic Pollutants. In: Large Marine Ecosystems: Status and trends. UNEP, Nairobi, pp. 165-176.
- ↑ Pivnenko, K., Eriksson, E. and Astrup, T. 2015. Waste paper for recycling: Overview and identification of potentially critical substances. Waste Manag. 45:134-42. doi: 10.1016/j.wasman.2015.02.028.
- ↑ Beyer, J., Song, Y., Tollefsen, K.E., Berge, J.A., Tveiten, L., Helland, A., Oxnevad, S. and Schoyen, M. 2022. The ecotoxicology of marine tributyltin (TBT) hotspots: A review. Marine Environmental Research 179, 105689
- ↑ Sharpe, R.M. and Skakkebaek, N.E. 1993. Are oestrogens involved in falling sperm counts and disorders of the male reproductive tract? Lancet 341, 1392–1395.
- ↑ Colborn, T. 1995. Pesticides—how research has succeeded and failed to translate science into policy: endocrinological effects on wildlife. Environ Health Perspect 103: 81–85.
- ↑ NSTC 1996. The health and ecological effects of endocrine disrupting chemicals. A framework for planning. Committee on Environment and Natural Resources, National Science and Technology Council (USA).
- ↑ Thomas K.V. and Hilton M.J. 2004. The occurrence of selected human pharmaceutical compounds in UK estuaries. Marine Pollution Bulletin 49: 436-444.
- ↑ Nihoul, C. and Ducrotoy, J-P. 1994. Impact of oil on the marine environment: Policy of the Paris commission on operational discharges from the offshore industry. Marine Pollution Bulletin 29: 323-329.
- ↑ Grant A. and Briggs A.D. 2002. Toxicity of sediments from around a North Sea oil platform: are metals or hydrocarbons responsible for ecological impacts? Marine Environmental Research 53: 95-116.
- ↑ Laane, R.W.P.M., Sonneveldt, H.L.A., Van der Weyden, A.J., Loch, J.P.G. and Groeneveld, G. 1999. Trends in the spatial and temporal distribution of metals (Cd, Cu, Zn and Pb) and organic compounds (PCBs and PAHs) in Dutch coastal zone sediments from 1981 to 1996: a model case study for Cd and PCBs. Journal of Sea Research 41: 1–17.
- ↑ Jones, E., Qadir, M., van Vliet, M.T.H., Smakhtin, V. and Kang, S-M. 2019. The state of desalination and brine production: A global outlook. Science of the Total Environment 657: 1343–1356
- ↑ Roberts, D.A., Johnston, E.L. and Knott, N.A. 2010. Impacts of desalination plant discharges on the marine environment: a critical review of published studies. Water Res. 44: 5117–5128
Please note that others may also have edited the contents of this article.
|