Difference between revisions of "TBT"
Dronkers J (talk | contribs) |
|||
| (37 intermediate revisions by 4 users not shown) | |||
| Line 1: | Line 1: | ||
| + | {{tocright}} | ||
{{ | {{ | ||
| − | Definition|title= tributyltin | + | Definition|title= tributyltin (TBT) |
| − | |definition= | + | |definition= Compound used in [[antifouling paints|antifouling paint]] for ships hulls which is toxic to marine life <ref>Sullivan P (ed.), 1999. Eric Sullivan's encyclopaedic dictionary. 6th edition. LPP Reference Publishing.</ref>.}} |
| − | <ref>Sullivan P (ed.), 1999. Eric Sullivan's encyclopaedic dictionary. 6th edition. LPP Reference Publishing.</ref>}} | ||
| − | + | ==Notes== | |
| − | == Notes == | ||
{| class="toccolours" border="1" style="float: right; clear: right; margin: 0 0 1em 1em; border-collapse: collapse;" | {| class="toccolours" border="1" style="float: right; clear: right; margin: 0 0 1em 1em; border-collapse: collapse;" | ||
| − | ! bgcolor="#FF8888" | Tributyltin | + | ! bgcolor="#FF8888" | Tributyltin |
|- | |- | ||
| − | | align="center" bgcolor="#FFFFFF" | [[Image: | + | | align="center" bgcolor="#FFFFFF" | [[Image:TBT.gif|200px|Tributyltin]] |
|- | |- | ||
! bgcolor="#8888FF" | Formula | ! bgcolor="#8888FF" | Formula | ||
|- | |- | ||
| − | | align="center" | C<sub> | + | | align="center" | C<sub>12</sub>H<sub>28</sub>Sn |
|- | |- | ||
|} | |} | ||
| − | [[ | + | TBT is an [[organotin compounds|organotin compound]], meaning it's an organic compound with an associated tin molecule. Of all organotins TBT is the one which in the past has caused most damage to the marine environment. |
| − | TBT concentrations ranged from 1 ng/l in | + | [[Antifouling paints]] containing TBT are much more effective and long lasting than mercury or copper based antifouling paints. They were first painted on ships in the 1970s and were subsequently also commonly used on net enclosures of mariculture installations<ref name = pol>Clark, R,B., 1999. Marine pollution. Oxford University press, Fourth edition, pp 161</ref>. |
| + | |||
| + | TBT concentrations ranged from 1 ng/l in boat free areas to levels above 600 ng/l near marinas. Even levels above 1 µg/l can be reached in heavily polluted marinas. Such concentrations, which are [[toxic]] for mollusk larvae, have caused [[recruitment]] failure of scallops and oysters. | ||
TBT also causes a variety of sublethal effects at much lower levels. Concentrations of 10 ng/l can cause reduced growth and shell thickening in oysters, which reduces the size of the animals inside, making the oysters unmarketable. | TBT also causes a variety of sublethal effects at much lower levels. Concentrations of 10 ng/l can cause reduced growth and shell thickening in oysters, which reduces the size of the animals inside, making the oysters unmarketable. | ||
| − | Another sublethal effect of TBT is the development of an nonfunctional penis in females, a condition called [[TBT and Imposex|imposex]]. The oviduct and the female genital opening are eventually overgrown by the developing penis and are blocked so that the eggs can not be laid. The affected populations decline and can eventually become locally extinct. | + | Another sublethal effect of TBT is the development of an nonfunctional penis in females, a condition called [[TBT and Imposex|imposex]]. The oviduct and the female genital opening are eventually overgrown by the developing penis and are blocked so that the eggs can not be laid. The affected populations decline and can eventually become locally extinct<ref name = pol>Clark, R,B., 1999. Marine pollution. Oxford University press, Fourth edition, pp 161</ref>. |
| + | Awareness of these effects resulted in an international ban to apply TBT on ships starting from 2003, and the obligation to remove all TBT on ships by 2008.<ref> | ||
| + | |||
| + | Maes, F.; Gonsaeles, G.; Le Roy, D.; Konings, F.; Vermeire, I. (2000). Beleidsvoorbereidend onderzoek inzake de implementatie van OSPAR-beslissingen en -aanbevelingen: eindrapport (offerte VMM.AK.1999-2). Maritiem Instituut Universiteit Gent: Gent. 50 + annexes pp.</ref> | ||
| + | |||
| + | Mammals, birds, crustatians and fish seem to be able to break down TBT. Therefore there is little or no risk for [[biomagnification]] of TBT<ref>Kennish, M. J. (1996): Practical Handbook of Estuarine and Marine Pollution, CRC Press 524 pp</ref>. | ||
| + | <P> | ||
| + | <BR> | ||
| + | <P> | ||
| + | |||
| + | ==Related articles== | ||
| + | :[[TBT and intersex in periwinkles]] | ||
| + | :[[Antifouling paints]] | ||
| + | :[[TBT and intersex in periwinkles]] | ||
| + | :[[Coastal pollution and impacts]] | ||
| + | :[[Endocrine disrupting compounds in the coastal environment]] | ||
| + | <P> | ||
| + | <BR> | ||
| + | <P> | ||
| + | == Environmental standards and legislation == | ||
| + | [[OSPAR List of priority substances|Included in the OSPAR list of substances of priority action]] | ||
| + | [[List of priority substances|Included in the water framework list of priority substances]] | ||
| + | <P> | ||
| + | <BR> | ||
| + | <P> | ||
== See also == | == See also == | ||
| − | + | ||
| + | [http://www.vliz.be/projects/endis/EDnorth.php?showchemprop=true&showeffects=true&chemeffects=true&chemid=327 TBT on the ED North Database] | ||
| + | |||
| + | [http://www.ospar.org/documents%5Cdbase%5Cpublications%5CP00103_BD%20on%20organotin.pdf OSPAR background document on organic tin compounds] | ||
| + | |||
[[TBT and Imposex]] | [[TBT and Imposex]] | ||
| + | <P> | ||
| + | <BR> | ||
| + | <P> | ||
| + | ==References== | ||
| + | <references/> | ||
| + | |||
| + | {{author | ||
| + | |AuthorID=19826 | ||
| + | |AuthorFullName=Daphnis De Pooter | ||
| + | |AuthorName=Daphnisd}} | ||
| + | |||
| + | [[Category:Toxicity chemicals]] | ||
Latest revision as of 15:57, 15 August 2024
Definition of tributyltin (TBT):
Compound used in antifouling paint for ships hulls which is toxic to marine life [1].
This is the common definition for tributyltin (TBT), other definitions can be discussed in the article
|
Notes
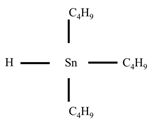
| Tributyltin |
|---|
|
| Formula |
| C12H28Sn |
TBT is an organotin compound, meaning it's an organic compound with an associated tin molecule. Of all organotins TBT is the one which in the past has caused most damage to the marine environment.
Antifouling paints containing TBT are much more effective and long lasting than mercury or copper based antifouling paints. They were first painted on ships in the 1970s and were subsequently also commonly used on net enclosures of mariculture installations[2].
TBT concentrations ranged from 1 ng/l in boat free areas to levels above 600 ng/l near marinas. Even levels above 1 µg/l can be reached in heavily polluted marinas. Such concentrations, which are toxic for mollusk larvae, have caused recruitment failure of scallops and oysters. TBT also causes a variety of sublethal effects at much lower levels. Concentrations of 10 ng/l can cause reduced growth and shell thickening in oysters, which reduces the size of the animals inside, making the oysters unmarketable. Another sublethal effect of TBT is the development of an nonfunctional penis in females, a condition called imposex. The oviduct and the female genital opening are eventually overgrown by the developing penis and are blocked so that the eggs can not be laid. The affected populations decline and can eventually become locally extinct[2]. Awareness of these effects resulted in an international ban to apply TBT on ships starting from 2003, and the obligation to remove all TBT on ships by 2008.[3]
Mammals, birds, crustatians and fish seem to be able to break down TBT. Therefore there is little or no risk for biomagnification of TBT[4].
Related articles
- TBT and intersex in periwinkles
- Antifouling paints
- TBT and intersex in periwinkles
- Coastal pollution and impacts
- Endocrine disrupting compounds in the coastal environment
Environmental standards and legislation
Included in the OSPAR list of substances of priority action
Included in the water framework list of priority substances
See also
OSPAR background document on organic tin compounds
References
- ↑ Sullivan P (ed.), 1999. Eric Sullivan's encyclopaedic dictionary. 6th edition. LPP Reference Publishing.
- ↑ 2.0 2.1 Clark, R,B., 1999. Marine pollution. Oxford University press, Fourth edition, pp 161
- ↑ Maes, F.; Gonsaeles, G.; Le Roy, D.; Konings, F.; Vermeire, I. (2000). Beleidsvoorbereidend onderzoek inzake de implementatie van OSPAR-beslissingen en -aanbevelingen: eindrapport (offerte VMM.AK.1999-2). Maritiem Instituut Universiteit Gent: Gent. 50 + annexes pp.
- ↑ Kennish, M. J. (1996): Practical Handbook of Estuarine and Marine Pollution, CRC Press 524 pp
Please note that others may also have edited the contents of this article.
|