Difference between revisions of "Effects of fisheries on marine biodiversity"
Dronkers J (talk | contribs) |
Dronkers J (talk | contribs) |
||
| Line 39: | Line 39: | ||
Sessile epibenthic species are most likely to be vulnerable to the passage of bottom gears. The disappearance of reefs of the calcareous tube building worm, ''Sabellaria spinulosa'' Leukart, and their replacement by small polychaete communities, indicated that dredging activity had caused measurable changes in the Wadden Sea benthic community<ref name="Riesen1982">Riesen, W. and Reise, K. 1982. Macrobenthos of the subtidal Wadden Sea: revisited after 55 years. Helgolander Meeresuntersuchungen 35: 409-423</ref>. | Sessile epibenthic species are most likely to be vulnerable to the passage of bottom gears. The disappearance of reefs of the calcareous tube building worm, ''Sabellaria spinulosa'' Leukart, and their replacement by small polychaete communities, indicated that dredging activity had caused measurable changes in the Wadden Sea benthic community<ref name="Riesen1982">Riesen, W. and Reise, K. 1982. Macrobenthos of the subtidal Wadden Sea: revisited after 55 years. Helgolander Meeresuntersuchungen 35: 409-423</ref>. | ||
| − | A particular sensitive hard-bottom habitat are the deep-water coral communities of the ''Lophelia pertusa'' reefs, see Fig. 1. These communities are mainly found at the offshore shelf edges of the [[Biodiversity_in_the_European_Seas#The_Arctic_Ocean.5B4.5D|Arctic]] and [[Biodiversity_in_the_European_Seas#The_North-east_Atlantic_Ocean.5B3.5D|North-Atlantic ocean]], see also the article [[ | + | A particular sensitive hard-bottom habitat are the deep-water coral communities of the ''Lophelia pertusa'' reefs, see Fig. 1. These communities are mainly found at the offshore shelf edges of the [[Biodiversity_in_the_European_Seas#The_Arctic_Ocean.5B4.5D|Arctic]] and [[Biodiversity_in_the_European_Seas#The_North-east_Atlantic_Ocean.5B3.5D|North-Atlantic ocean]], see also the article [[Dynamics, threats and management of biogenic reefs]]. Some offshore reefs have experienced considerable damage due to trawling activities. The ''Lophelia'' reefs have since 1999 been protected from fishing activities by the Norwegian authorities<ref name="EEA2002">EEA 2002. Europe’s biodiversity – biogeographical regions and seas – biogeographical regions in Europe – The Arctic Ocean. EEA report 1/2002</ref>. |
Revision as of 18:01, 7 March 2023
Fishing is the most widespread human exploitative activity in the marine environment. Pauly and Christenen (1995[1]) estimated that over 20 % of the marine primary production is required to sustain fisheries in many intensively fished coastal ecosystems.
Fishing has a number of direct effects on marine ecosystems because it is responsible for increasing mortality of target and by-catch species; an important physical impact on the habitat of benthic organisms is caused by bottom trawling. The direct effects of fishing have indirect implications for other species as well. Fisheries remove prey that piscivorous fishes, birds and mammals would otherwise consume, or may remove predators that would otherwise control prey populations. Reductions in the density of some species may affect competitive interactions and result in the proliferation of non-target species. The activities of fisheries also favour scavengers, they obtain more food by the discarded by-catch and because a range of species are killed, but not retained by towed gears[2].
Contents
Effects of fishing on target species
Global landing of fish and other marine catches began stagnating in the early 1980s[3]. Biomass of large predatory fish species in the North Atlantic fell by 90 % during the twentieth century, leading to declines of catches throughout the North Atlantic, notably in eastern Canada. It has taken less than a century for North Atlantic fisheries to reduce the biomass of the high-trophic-level fishes to under 10% of their original amounts[4].
Historically, fishing started at the top of most food chains by removing the highly valuable and more easily cacheable species, then moved down to the next biggest species as those above were depleted and were no longer easily or economically caught. The downward shift towards fish catches of lower trophic levels results in ‘fishing down the food web’. The mean trophic level of reported catches had declined over the years. For all marine areas, the trend has been a decline in the mean trophic level of the fisheries landings form slightly more than 3.3 in the early 1950s to less than 3.1 in 1994[5]. See also the article Overexploitation.
Another shift in the global landings of fishes in the last 50 year is from shallow to deeper water species; this resulted in the fact that the mean longevity of the fish species caught, has increased dramatically. This trend is a serious concern because species with larger body size, longer life span, later sexual maturity and slow growth (e.g. Dogfish Scyliorhinus canicula, Rays, Conger eel Conger conger) are more vulnerable to overfishing[6].
Effects on by-catch species
Benthic organisms and other unwanted by-catch are often discarded and a range of species are killed, but not retained by towed gears.
Some by-catch species have been affected dramatically by fishing. For example, the sizes of three dolphin populations (a Stenella longirostris population, a S. attenuata population and a second S. longirostris population) caught by tuna boats in the eastern tropical Pacific were reduced to 20%, 35-50% and 58-72% of pre-exploitation levels by 1997[2].
Effects of physical disturbance
Bottom trawling
According to the Millennium Ecosystem Assessment (2005[7]), seabed damage is one of the predominant drivers for biodiversity loss in coastal ecosystems world-wide. Disruptive fishing techniques, including bottom trawling, are considered among the major causes of physical destruction of marine coastal habitats at global scales. It is estimated that about 75% of the global continental shelf is affected by trawling and dredging activities[8].
The direct effects include the scraping, scouring and resuspension of the substratum. The resuspension, transport and subsequent deposition of sediment may affect the settlement and feeding of the biota in other areas[2]. The effects of sediment resuspension include the release of nutrients held in the sediment, exposure of anoxic layers, release of contaminants, increasing biological oxygen demand, smothering of feeding and respiratory organs and reduction of light availability[9]. Sediment deposition has been shown to inhibit the settlement and growth of oysters and scallops[10][11].
Structurally complex habitats (such as seagrass meadows, biogenic reefs) and those that are relatively undisturbed by natural perturbations (e.g. deep-water mud substrata) are more adversely affected than unconsolidated sediment habitats that occur in shallow coastal waters. For example, in shallow turbid regions such as the southern North Sea, fauna are adapted to continual disturbance by wave and tidal action and therefore more resilient to the effects of trawling[8]. In soft mud communities a large proportion of the fauna live in burrows up to 2 m deep that make them less sensitive to passing trawls[12]. In general, habitat recovery is relatively rapid (days to several months) for sandy habitats dominated by physical processes, while muddy sandy habitats mediated by a combination of physical, chemical and biological processes require more time for recovery (months-years). Slow-growing biota with large biomass, such as sponges and soft corals, take much longer to recover (up to 8 years) than biota with a shorter lifespan, such as polychaetes (<1 year) [13]. Chronic fishing disturbance affect the epifauna by modification of substrata and removal of biogenic concretions and a consequent decline in the abundance of fauna associated with them. The loss of biogenic species not only reduces the supply of important prey species, but also increases predation risk for juvenile commercial species thereby lowering subsequent recruitment to the adult stocks[14]. Conversely, scavengers and small-bodied organisms, such as polychaete worms, dominate heavily fished areas[8]. Major changes in habitat can lead to changes in the composition of the resident fish fauna. Changes attributed to the fisheries are also identified in the mesozooplankton composition. For instance, the mesozooplankton taken in continuous plankton recorder samples in the central North Sea were numerically dominated by calanoid copepods from 1958 to the late 1970s, whereas samples taken from the same stations from the early 1980s to early 1990s were dominated by the pluteus larvae of echinoid and ophiuroid echinoderms. This trend is consistent with the reported increases in the abundance of echinoderms in benthic communities which may have been stimulated, in part, by bottom trawling[15].
While the effects of physical disturbance are short-lived in communities adapted to frequent natural perturbations (e.g. a cockle community), the effects are long-lived in communities of habitats exposed to fewer disturbances (e.g. the abyssal plane)[2]. Changes are also relatively short-lived for the majority of small species, while longer-lived organisms recolonize more slowly after disturbance. For example, Beukema (1995[16]) reported that the biomass of gaper clams, Mya arenaria L., took 2 years to recover after lugworm dredging in the Wadden Sea, whereas small polychaetes and bivalves had recolonized the dredged areas within 12 months. Communities dominated by long-lived suspension feeders are most likely to be replaced by a community of opportunistic deposit-feeding species and mobile epifauna when subjected to large-scale and intense fishing disturbance. More dramatically, biogenic structures that increase the complexity of the epibenthic habitat (e.g. worm tubes) create specialized environmental conditions by altering local hydrographic conditions that encourage the development of a specialized associated community. Loss of such structures will also affect the survivorship of any associated species and prolong the recolonisation process.[2].
Sessile epibenthic species are most likely to be vulnerable to the passage of bottom gears. The disappearance of reefs of the calcareous tube building worm, Sabellaria spinulosa Leukart, and their replacement by small polychaete communities, indicated that dredging activity had caused measurable changes in the Wadden Sea benthic community[17]. A particular sensitive hard-bottom habitat are the deep-water coral communities of the Lophelia pertusa reefs, see Fig. 1. These communities are mainly found at the offshore shelf edges of the Arctic and North-Atlantic ocean, see also the article Dynamics, threats and management of biogenic reefs. Some offshore reefs have experienced considerable damage due to trawling activities. The Lophelia reefs have since 1999 been protected from fishing activities by the Norwegian authorities[18].

Similarly, the results of a trawl depletion experiment in the interreef areas of the Great Barrier Reef showed that each trawl removed and caught between 5 and 20% of the available biomass of sessile fauna, with 70-90% removed after 13 trawls[19].
Bottom trawling is not limited to the shelf seas; this fishing method is also practiced on the deeper continental slope. An example are chronically trawled parts of the continental slope of the north-western Mediterranean Sea, where important effects have been observed at depths between 200 and 800 m[20]. These effects include significant decreases in organic matter content (up to 52%), slower organic carbon turnover ( approx. 37%), and reduced meiofauna abundance (80%), biodiversity (50%), and nematode species richness (25%). The authors[20] caution that ultimately, intensive and chronic bottom trawling is deemed to transform large portions of the deep continental slope into faunal deserts and highly degraded seascapes.
The effects of seabed distubance vary according to the gears used and the habitats fished[2]. The heaviest damage is caused by scallop dredges, see Fig. 2. Both deposit- and suspension-feeders are vulnerable to scallop dredging across gravel, sand and mud habitats, but biogenic habitats suffer most[13]. Beam trawling can also be highly destructive, but this strongly depends upon habitat type.
Static bottom gears are anchored to the seabed and left to fish passively. The most commonly used are gill, trammel or tangle nets, which are designed to capture target species by enmeshing or tangling them[21][22] (see also Sampling tools for the marine environment). Because net and pot fisheries are static, the areas of seabed affected by each gear is insignificant compared with the widespread effects of mobile fishing gears. However, effects may be significant if concentrated in relatively small areas with communities of long-lived fauna (e.g. the foliose bryozoan Pentapora foliacea).
Other destructive fishing practices

Highly destructive fishing methods, using explosives or poison (cyanide), are widely practiced on the coral reefs of southeast Asia. About one third of the world's coral reefs is situated in southeast Asia, covering nearly 100,000 km2. It is estimated that 56% of these reefs are threatened by destructive fishing methods[23], see Fig. 3.
Poison (sodium cyanide) is used to stun fish (especially high-value aquarium fishes), making them easier to catch. The poison attacks the corals that die from repeated exposure; other organisms of the coral ecosystem suffer the same fate.

The use of explosives (potassium nitrate) not only kills the fish swimming around, but also severely damages the coral (Fig. 4). Regularly bombed reefs exhibit 50–80 percent coral mortality. Fish that are not captured float to the surface or sink to the bottom. The fishermen themselves risk their lives or lose limbs from prematurely exploding bombs.
While these destructive fishing methods are banned in most countries, enforcement is a major problem. The precariousness of the coastal population and lack of education are important drivers. The yields from these fishing methods are more than ten times the costs of poison and explosives. Due to the illegality of these practices, the extent is not well known.
Effects of ghost-fishing
When nets or catch-pots are lost, they may continue to fish. This phenomenon is known as ‘ghost-fishing’. Richardson et al. (2019[24]) estimate that 5.7% of all fishing nets, 8.6% of all traps, and 29% of all lines are lost around the world each year. In circumstances where nets or pots are snagged onto rocks, holding the net in place, or lost in relatively stable deep water environments, they may continue to fish indefinitely. In these cases, a typical pattern of capture is observed. Over the first few days, catches decline almost exponentially as the increasing weight of catch causes the net to collapse. Then, for the next few weeks, the decaying bodies of fishes and Crustacea attract large number of scavenging crustaceans, many of which are valuable commercial species and also become entangled in the net. Thereafter, there appears to be a continuous cycle of capture, decay and attraction for as long as the net has some entanglement properties[25]. Biodegradable gillnets have been developed with a shorter lifespan in the marine environment than conventional nylon nets. These nets shorten ghost fishing time by several years, which may outweigh the disadvantage of reduced catch efficiency over time[26].
Trophic cascading effect
Changes in one level of a food web can also have cascading effects on others. For example in the Black Sea, a trophic cascade was triggered by fishery removals of apex predators (bonito Sarda sarda, mackerel Scomber scombrus , bluefish Pomatomus saltatrix, dolphins), which caused a decreased consumer control and lead to higher abundance of planktivorous fish (i.e. Black Sea sprat Clupeonella cultriventris, anchovy Engraulis encrasicholus, horse mackerel Trachurus mediterraneus ponticus). The increased consumption by planktivorous fish caused a decline in zooplankton biomass that in turns allowed phytoplankton to increase. This chain of events is thought to explain the explosions of phytoplankton and jellyfish reported in the Black Sea over the past 30 years[27]. See the article Trophic cascade for other examples.
Food-web competition
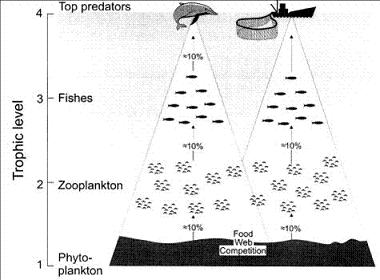
An apex predator may be affected by fisheries even when the prey and species caught do not overlap. This has been termed ‘food-web competition’[28]. Food-web competition occurs when there is potential overlap of the trophic flows supporting a given group (e.g. marine mammals) with the trophic flows supporting another group (e.g. fisheries), see Fig. 5. The relationship between the size of fishery catches and the amounts of primary production required to sustain fisheries and marine mammals suggests that the primary production available to marine mammals may decline as catches increase[4]
Effects on phenotypic evolution
Large changes in size-at-age and age-at-maturation of commercially exploited fish have been reported in a number of ecosystems. Survival and reproduction are functions of body size. Small fish generally incur higher mortality rates and produce fewer eggs than larger fish.
Gear is designed to remove some kinds of individuals in preference to others, usually individuals that are larger and, indirectly, older. The location of fishing is often non-random relative to spatial distributions of stocks, being concentrated where the harvestable biomass is greatest or where fishes are most accessible, or both. Fishing mortality is therefore selective with respect both to species and to phenotypic variation within species[29][30][31]. Moreover, even a uniform change in overall mortality is evolutionarily selective. This happens because mortality discounts fitness gains incurred in late life; traits such as maturation that involve trade-offs between fitness gains in early and late life are therefore affected by a change in mortality, selective or not.
There are strong indications that the observed changes have partly a genetic basis. However, it is difficult to distinguish genetic evolution from phenotypic plasticity, i.e., a tendency of phenotypic traits to take different values depending on the current environmental conditions. Rijnsdorp (1993[32]) carried out a study to disentangle the causes of a major phenotypic change in maturation of North Sea plaice (Pleuronectes platessa). He concluded that a substantial part of the change in maturation is consistent with genetic change caused by fishing. Simply through the action of fishing, fisheries generate selection, causing evolution in life-history traits. More recently, similar results have been obtained for many different fish populations[33][34], although the extent to which the documented changes are genetic versus environmental remains debated[35].
The common trend is a decreased age-at-maturation in heavily exploited fish stocks[33][34], but this selection pattern is not always consistent. For instance, there are two spatially separated fisheries targeting cod (Gadus morhua) in the Barents Sea: a feeder (exploitation of the stock on the feeding grounds) and a spawner fishery (exploitation of the stock on the spawning grounds). Fishing confined to the spawning grounds gives an advantage to late maturation. This is because the extra mortality due to fishing on the spawning grounds makes it advantageous to grow for longer before maturation and thereby to produce more eggs when risking a visit to the spawning ground. If fishing mortality on the feeding grounds is added on, the relatively small advantage to late maturation is changed to a large advantage to early maturation[36][37]. Remaining on the feeding grounds is now itself risky, and a fish that does not mature until about, say 8 years of age is most likely to be caught before it spawns.
Current patterns of fishing generate strong selection for early maturation and, given appropriate genetic variation, substantial genetic change can be expected. But, if one were to try to reverse the process by closing the fishery, selection for later maturation would be weak. In other words, it could be hard to undo the effects of inadvertent selection caused by fishing[38]
See also
References
- ↑ Pauly, D. and Christensen, V. 1995. Primary production required to sustain global fisheries. Nature 374: 255-257
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 Jennings, S. and Kaiser, M. 1998. The effects of fishing on marine ecosystems. Adv. Mar. Biol. 34: 201-352
- ↑ Watson, R. and Pauly, D. 2001. Systematic distortion in world fisheries catch trends. Nature 414 (6863): 534-536
- ↑ 4.0 4.1 Christensen, V., Guénette, S., Heymans, J.J. et al. 2003. Hundred-year decline of North Atlantic predatory fishes. Fish and Fisheries 4: 1-24 Cite error: Invalid
<ref>tag; name "" defined multiple times with different content - ↑ Pauly, D., Christenen, V., Dalsgaard, J., Froese, R. and Torres, F. Jr. 1998. Fishing Down Marine Food Webs. Science 279: 860-863
- ↑ Morato, T., Watson, R., Pitcher, T. J. and Pauly, D. 2006. Fishing down the deep. Fish and fisheries 7: 24-34
- ↑ Agardy, T. and Alder, J. (lead authors) 2005. Millennium Ecosystem Assessment Chapter 19 Coastal Systems. Washington, D.C., Island Press
- ↑ 8.0 8.1 8.2 Kaiser, M.J., Collie, J.S., Hall, S.J., Jennings, S., Poiner, I.R. 2002. Modification of marine habitats by trawling activities: prognosis and solutions. Fish Fisheries 3: 114–136
- ↑ Rhoads, D.C. 1974. Organism-sediment relations on the muddy sea floor. Oceanography and Marine Biology Annual Review 12: 263-300
- ↑ Moore, P.G 1977. Inorganic particulate suspensions in the sea and their effects on marine animals. Oceanography and Marine Biology Annual Review 15: 225-363
- ↑ Jones, J.B. 1992. Environmental impact of trawling on the seabed: a review. New Zealand Journal of Marine and Freshwater research 26: 59-67
- ↑ Atkinson, R.J.A. and Nash, R.D.M. 1990. Some preliminary observations on the burrows of Callianassa subterranean (Montagu) (Decapoda: thalassinidae) from the west coast of Scotland. Journal of Natural History 24: 403-413
- ↑ 13.0 13.1 Kaiser,M.J., Clarke, K.R., Hinz, H., Austen,M.C.V., Somerfield, P.J., Karakassis, I. 2006. Global analysis of response and recovery of benthic biota to fishing. Mar. Ecol. Prog. Ser. 311: 1–14
- ↑ Walters, C.J. and Juanes, F. 1993. Recruitment limitations as a consequence of natural selection for use of restricted feeding habitats and predation risk taking by juvenile fishes. Canadian Journal of Fisheries and Aquatic Science 50: 2058-2070
- ↑ Lindley, J.A., Gamble, J.C. and Hunt, H.G. 1995. A change in the zooplankton of the central North Sea (55° to 58°N): a possible consequence of changes in the benthos. Marine Ecology Progress Series 119: 299-303
- ↑ Beukema, J.J. 1995. Long-term effects of mechanical harvesting of lugworms Arenicola marina on the zoobenthic community of a tidal flat in the Wadden Sea. Netherlands Journal of Sea Research 33: 219-227
- ↑ Riesen, W. and Reise, K. 1982. Macrobenthos of the subtidal Wadden Sea: revisited after 55 years. Helgolander Meeresuntersuchungen 35: 409-423
- ↑ EEA 2002. Europe’s biodiversity – biogeographical regions and seas – biogeographical regions in Europe – The Arctic Ocean. EEA report 1/2002
- ↑ Poiner, I.R., Blaber, J.M., Brewer, D.T., Burridge, C., Caeser, D., Connell, M., Dennis, D., Dews, G.D., Ellis, N., Farmer, M., Glaister, J., Gribble, N., Hill, B.J., O’Connor, R., Milton, D.A., Pitcher, R., Salini, J.P., Taranto,T., Thomas, M., Toscas, P.,Wang, Y.G., Veronise, S. and Wassenberg,T.J. 1998. Final report on the effects of prawn trawling in the far northern section of the Great Barrier Reef. CSIRO Marine Laboratories
- ↑ 20.0 20.1 Pusceddu, A., Bianchelli, S., Martín, J., Puig, P., Palanques, A., Masqué, P. and Danovaro, R. 2014. Chronic and intensive bottom trawling impairs deep-sea biodiversity and ecosystem functioning. PNAS 111: 8861–8866
- ↑ Miller, R.J. 1977. Resource underutilization in a spider crab industry. Fisheries 2: 9-13
- ↑ Potter, E.C.E. and Pawson, M.G. 1991. Gill netting. Laboratory leaflets, MAFF, Directorate of Fisheries Research, Lowestoft 69, 34pp.
- ↑ 23.0 23.1 23.2 Burke, L., Selig, E. and Spalding, M. 2002. Reefs at risk in southeast Asia. World Resources Institute. ISBN 1-56973-490-9
- ↑ Richardson, K., Hardesty, B.D. and Wilcox, C. 2019. Estimates of fishing gear loss rates at a global scale: A literature review and meta‐analysis, Fish and Fisheries 20(6): 1218-1231
- ↑ Carr, H.A., Blott, A.J. and Caruso, P.G. 1992. A study of ghost gillnets in the inshore waters of southern New England. In “MTS” 92: Global Ocean Partnership, pp. 361-367. Marine Technology Society, Washington D.C.
- ↑ Brakstad, O.G., Sorensen, L., Hakvag, S., Fore, H.M., Su, B., Aas, M., Ribicic, D. and Grimaldo, E. 2022. The fate of conventional and potentially degradable gillnets in a seawater-sediment system. Marine Pollution Bulletin 180, 113759
- ↑ Daskalov, G.M. 2002. Overfishing drives a trophic cascade in the Black Sea. Marine Ecology Progress Series 225: 53-63
- ↑ 28.0 28.1 Trites, A., Christensen, V. and Pauly, D. 1997. Competition between fisheries and marine mammals for prey and primary production in the Pacific Ocean. Journal North West Atlantic Fisheries Science 22: 173-187
- ↑ Stokes, T.K., McGlade, J.M. and Law, R. (eds) 1993. The exploitation of Evolving Resources. Lecture Notes in Biomathematics, 99. Springer-Verlag, Berlin. 264 pp.
- ↑ Law, R. 2000. Fishing, selection, and phenotypic evolution. ICES Journal of Marine Science 57: 659-668
- ↑ Jennings, S., Reynolds, J.D. and Mills, S.C. 1998. Life history correlates of responses to fisheries exploitation. Proceedings of the Royal Society London series B 265: 333-339
- ↑ Rijnsdorp, A.D. 1993. Fisheries as a large-scale experiment on life-history evolution: disentangling phenotypic and genetic effects in changes in maturation and reproduction of North Sea plaice, Pleuronectes platessa L. Oecologia 96: 391-401
- ↑ 33.0 33.1 Jørgensen, C., Enberg, K. Dunlop, E.S. et al. 2007. Managing evolving fish stocks. Science 318: 1247-1248
- ↑ 34.0 34.1 Heino, M. and Dieckmann, U. 2008. Detecting fisheries-induced life-history evolution: an overview of the reaction-norm approach. Bulletin of Marine Science 83: 69-93
- ↑ Marshall, C. T. and H. I. Browman, eds. 2007. Disentangling the causes of maturation trends in exploited fish populations. Marine Ecology-Progress Series 335: 249–310
- ↑ Borisov, V.M. 1978. The selective effect of fishing on the population structure of species with a long life cycle. Journal of Ichthyology 18: 896-904
- ↑ Law, R. and Grey, D.R. 1989. Evolution of yields from populations with age-specific cropping. Evolutionary Ecology 3: 343-359
- ↑ Heino, M., Pauli, B.D. and Dieckmann, U. 2015. Fisheries-Induced Evolution. Annual Review of Ecology, Evolution, and Systematics 46: 461-480
Please note that others may also have edited the contents of this article.
|
