Difference between revisions of "Coral Molecular Biomarkers"
(→An Overview of the Ecotoxicology of Coral) |
Dronkers J (talk | contribs) |
||
| (12 intermediate revisions by 3 users not shown) | |||
| Line 1: | Line 1: | ||
== An Overview of the Ecotoxicology of Coral == | == An Overview of the Ecotoxicology of Coral == | ||
| + | |||
| + | === Abstract === | ||
| + | Coral reefs worldwide are undergoing serious threats from anthropogenic pressures. Traditionally, coral reef assessment and monitoring programs use mortality, such as percent death or species loss, as an indicator, which is not an effective metric for heath. Coral ecotoxicology has emerged to apply biomedical techniques to ‘diagnose’ coral heath at a sublethal level. In order to protect coral reefs from further damages, it is critical to detect biological stress responses at the early stages so that the process may be reversed. Increasing numbers of coral ecotoxicological studies have shown the effective use of such techniques in the past decade. This review provides overview of molecular biomarkers used in coral ecotoxicological studies to detect sublethal responses in reef-building, scleractinian coral. We summarize the major cellular stress-response pathways characterized in corals, as well as the effects of major stress-inducing agents on coral are discussed. | ||
| + | |||
=== I. Introduction === | === I. Introduction === | ||
| Line 16: | Line 20: | ||
CYP, GST and MXR are the primary biomarkers to assess the xenobiotic response in coral. Most studies use all of these biomarkers to ensure the presence of the response (Downs et al. 2012; Downs et al. 2009; Downs & Downs 2006; Downs et al. 2006; Rougée et al. 2006). Table 1 summarizes the biomarkers that are used for assessing the xenobiotic response in coral. | CYP, GST and MXR are the primary biomarkers to assess the xenobiotic response in coral. Most studies use all of these biomarkers to ensure the presence of the response (Downs et al. 2012; Downs et al. 2009; Downs & Downs 2006; Downs et al. 2006; Rougée et al. 2006). Table 1 summarizes the biomarkers that are used for assessing the xenobiotic response in coral. | ||
| − | [[File:CoralTox Fig1.jpg | + | [[File:CoralTox Fig1.jpg|thumbnail|Figure 1]] |
[[File:Coral Ecotoxicology Table1.jpg|thumbnail|Table 1 List of biomarkers for xenobiotic response in coral]] | [[File:Coral Ecotoxicology Table1.jpg|thumbnail|Table 1 List of biomarkers for xenobiotic response in coral]] | ||
| Line 71: | Line 75: | ||
[[File:Coral Ecotox Table6.jpg|thumbnail|Table 6]] | [[File:Coral Ecotox Table6.jpg|thumbnail|Table 6]] | ||
| − | |||
=== III. Responses of coral to specific stressors: === | === III. Responses of coral to specific stressors: === | ||
| Line 80: | Line 83: | ||
===== Copper (Cu): ===== | ===== Copper (Cu): ===== | ||
Copper (Cu) is an essential element to all life, but can be toxic to some marine organisms, including coral, even at relatively low concentrations (Victor & Richmond 2005). Cu occurs naturally in the marine environment. Its concentrations in seawater are reported to be 0.01 - 0.03 μg/l in relatively pristine marine environments (Victor & Richmond, 2005), but it could range up to 50 μg/l at highly polluted sites (Mitchelmore et al. 2007; Reichelt-Brushett & Harrison 1999). Anthropogenic sources of Cu are diverse: Cu is found in antifouling paints, sewer discharge, fungicides, herbicides, construction materials of waterfront structures, and heat exchangers in power plants (Victor & Richmond 2005). | Copper (Cu) is an essential element to all life, but can be toxic to some marine organisms, including coral, even at relatively low concentrations (Victor & Richmond 2005). Cu occurs naturally in the marine environment. Its concentrations in seawater are reported to be 0.01 - 0.03 μg/l in relatively pristine marine environments (Victor & Richmond, 2005), but it could range up to 50 μg/l at highly polluted sites (Mitchelmore et al. 2007; Reichelt-Brushett & Harrison 1999). Anthropogenic sources of Cu are diverse: Cu is found in antifouling paints, sewer discharge, fungicides, herbicides, construction materials of waterfront structures, and heat exchangers in power plants (Victor & Richmond 2005). | ||
| − | In adult coral, Cu is reported to accumulate in tissues (and the symbiont algal tissues), and Cu exposure induces oxidative stress response. Even exposure at low Cu concentrations (≦5 μg/l) of P. damicornis in laboratory experiments resulted in a significantly elevated level of GSH (Mitchelmore et al. 2007). A 14-day 50 μg/l of Cu exposure resulted in 100% mortality of P. damicornis (Mitchelmore et al. 2007). Furthermore, Bielmyer et al. (2010) reported significant decreases in skeletal growth in A. cervicornis and P. damicornis. Interestingly, the relationships between physiological/toxicological endpoints and Cu accumulation differed among 3 coral species they studied, which indicates the presence of interspecific differences in sensitivity to Cu. | + | In adult coral, Cu is reported to accumulate in tissues (and the symbiont algal tissues), and Cu exposure induces oxidative stress response. Even exposure at low Cu concentrations (≦5 μg/l) of <i>P. damicornis</i> in laboratory experiments resulted in a significantly elevated level of GSH (Mitchelmore et al. 2007). A 14-day 50 μg/l of Cu exposure resulted in 100% mortality of <i>P. damicornis</i> (Mitchelmore et al. 2007). Furthermore, Bielmyer et al. (2010) reported significant decreases in skeletal growth in <i>A. cervicornis</i> and <i>P. damicornis</i>. Interestingly, the relationships between physiological/toxicological endpoints and Cu accumulation differed among 3 coral species they studied, which indicates the presence of interspecific differences in sensitivity to Cu. |
Cu is known to affect not only coral but also its symbiotic algae, and the reduction in photosynthetic levels, as well as bleaching, has been reported (Negri & Hoogenboom 2011). However, one experiment shows that Cu induced coral bleaching without affecting the algal photosynthesis, thereby confirming that Cu directly affects corals (Jones 2004). Bielmyer et al. (2010) also reported that no changes in zooxanthellae communities were apparent in response to increasing Cu concentrations. | Cu is known to affect not only coral but also its symbiotic algae, and the reduction in photosynthetic levels, as well as bleaching, has been reported (Negri & Hoogenboom 2011). However, one experiment shows that Cu induced coral bleaching without affecting the algal photosynthesis, thereby confirming that Cu directly affects corals (Jones 2004). Bielmyer et al. (2010) also reported that no changes in zooxanthellae communities were apparent in response to increasing Cu concentrations. | ||
Coral larvae and juveniles show high sensitivity to Cu. Laboratory experiments show that Cu inhibits fertilization success (Victor & Richmond 2005; Reichelt-Brushett & Harrison 1999), survival of gametes (Victor & Richmond 2005), metamorphosis of larvae (Negri & Hoogenboom 2011), and larval settlement & survival (Reichelt-Brushett & Harrison 2000). According to Reichelt-Brushett & Harrison (2000), Cu inhibits the metamorphosis of coral larvae at lower concentrations than any metal tested so far. | Coral larvae and juveniles show high sensitivity to Cu. Laboratory experiments show that Cu inhibits fertilization success (Victor & Richmond 2005; Reichelt-Brushett & Harrison 1999), survival of gametes (Victor & Richmond 2005), metamorphosis of larvae (Negri & Hoogenboom 2011), and larval settlement & survival (Reichelt-Brushett & Harrison 2000). According to Reichelt-Brushett & Harrison (2000), Cu inhibits the metamorphosis of coral larvae at lower concentrations than any metal tested so far. | ||
| Line 101: | Line 104: | ||
===== Zinc (Zn): ===== | ===== Zinc (Zn): ===== | ||
| − | Zinc is an essential element for all life, and Zn requirements for organisms are usually higher than for Cu (Reichelt-Brushett & Harrison 2004). The average Zn concentration recorded in the world’s oceans is approximately 5 μg/l, although much higher amounts are recorded in sewage and industrial waste discharge (Reichelt-Brushett & Harrison 1999). The results from a laboratory exposure experiment of G. aspera gametes to Zn did not show any significant reduction in fertilization success up to 500 μg/l of Zn concentrations (Reichelt-Brushett & Harrison 1999). However, a reduction in the fertilization success of Favites chinensis and Platygyra ryukyuensis was previously reported by Heyward (1988) at 500-1000 μg/l of Zn. According to | + | Zinc is an essential element for all life, and Zn requirements for organisms are usually higher than for Cu (Reichelt-Brushett & Harrison 2004). The average Zn concentration recorded in the world’s oceans is approximately 5 μg/l, although much higher amounts are recorded in sewage and industrial waste discharge (Reichelt-Brushett & Harrison 1999). The results from a laboratory exposure experiment of <i>G. aspera</i> gametes to Zn did not show any significant reduction in fertilization success up to 500 μg/l of Zn concentrations (Reichelt-Brushett & Harrison 1999). However, a reduction in the fertilization success of <i>Favites chinensis</i> and <i>Platygyra ryukyuensis</i> was previously reported by Heyward (1988) at 500-1000 μg/l of Zn. According to Reichelt-Brushett & Harrison (1999), the concentrations of Zn used in their study were relatively low and the exposure time was short (5-hour), suggesting that exposure at higher concentrations or for longer periods of time may be necessary to detect the effects. Additionally, there seem to exist interspecific differences in sensitivities not only to Zn, but also to Cu and Cd. Therefore, generalizing from one species to another may be difficult for certain stressors (Reichelt-Brushett & Harrison 1999; Heyward 1988). Further studies are needed to conclude the effects of Zn on coral fertilization, and to find out the interspecific differences in sensitivity to Zn. |
===== Metal accumulation in coral as a pollution indicator: ===== | ===== Metal accumulation in coral as a pollution indicator: ===== | ||
| Line 110: | Line 113: | ||
===== TBT: ===== | ===== TBT: ===== | ||
| − | Due to their toxicity, antifouling paints that contain organotin compounds, such as tributylin (TBT), have been banned by the International Maritime Organization (IMO). Applying or reapplying paints with TBT has been banned since 2003, yet as of 2008 only 34 out of 168 nations have ratified the Convention on the Control of Harmful Anti-fouling Systems on Ships (International Maritime Organization 2012). Therefore, TBT still poses a threat to marine organisms. Furthermore, this compound is stored in sediments and re-enters the food chain when the sea bottom is stirred up (Adamson 2003). Effects of organotin (TBT) are relatively well studied in coral, and have been shown to affect their entire life history, such as reduction in fertilization, inhibition of larval settlement and metamorphosis, and bleaching in juveniles as well as in adults (Negri & Marshall 2009; Inoue et al. | + | Due to their toxicity, antifouling paints that contain organotin compounds, such as tributylin (TBT), have been banned by the International Maritime Organization (IMO). Applying or reapplying paints with TBT has been banned since 2003, yet as of 2008 only 34 out of 168 nations have ratified the Convention on the Control of Harmful Anti-fouling Systems on Ships (International Maritime Organization 2012). Therefore, TBT still poses a threat to marine organisms. Furthermore, this compound is stored in sediments and re-enters the food chain when the sea bottom is stirred up (Adamson 2003). Effects of organotin (TBT) are relatively well studied in coral, and have been shown to affect their entire life history, such as reduction in fertilization, inhibition of larval settlement and metamorphosis, and bleaching in juveniles as well as in adults (Negri & Marshall 2009; Inoue et al. 2004; Negri et al. 2002). |
===== Irgarol: ===== | ===== Irgarol: ===== | ||
| Line 170: | Line 173: | ||
* Markey, K.L. et al., 2007. Insecticides and a fungicide affect multiple coral life stages. Marine Ecology Progress Series, 330, pp.127–137. | * Markey, K.L. et al., 2007. Insecticides and a fungicide affect multiple coral life stages. Marine Ecology Progress Series, 330, pp.127–137. | ||
* Mitchelmore, C.L., Verde, E.A. & Weis, V.M., 2007. Uptake and partitioning of copper and cadmium in the coral Pocillopora damicornis. Aquatic Toxicology, 85(1), pp.48–56. | * Mitchelmore, C.L., Verde, E.A. & Weis, V.M., 2007. Uptake and partitioning of copper and cadmium in the coral Pocillopora damicornis. Aquatic Toxicology, 85(1), pp.48–56. | ||
| − | * Negri, A. & Marshall, P., | + | * Negri, A. & Marshall, P., 2009. TBT contamination of remote marine environments: Ship groundings and ice-breakers as sources of organotins in the Great Barrier Reef and Antarctica. Journal of Environmental Management, 90: S31–S40. |
* Negri, A.P. & Heyward, A.J., 2001. Inhibition of coral fertilisation and larval metamorphosis by tributyltin and copper. Marine Environmental Research, 51(1), pp.17–27. | * Negri, A.P. & Heyward, A.J., 2001. Inhibition of coral fertilisation and larval metamorphosis by tributyltin and copper. Marine Environmental Research, 51(1), pp.17–27. | ||
* Negri, A.P. & Heyward, A.J., 2000. Inhibition of fertilization and larval metamorphosis of the oral Acropora millepora by Petroleum Products. Marine pollution bulletin, 41(7), pp.420–427. | * Negri, A.P. & Heyward, A.J., 2000. Inhibition of fertilization and larval metamorphosis of the oral Acropora millepora by Petroleum Products. Marine pollution bulletin, 41(7), pp.420–427. | ||
| Line 198: | Line 201: | ||
* Spokes, L. & Croot, P., 2003. Iron in the oceans. Environmental Science Published for Everybody Roudn the Earth. Available at: http://www.atmosphere.mpg.de/enid/1vv.html [Accessed November 6, 2012]. | * Spokes, L. & Croot, P., 2003. Iron in the oceans. Environmental Science Published for Everybody Roudn the Earth. Available at: http://www.atmosphere.mpg.de/enid/1vv.html [Accessed November 6, 2012]. | ||
* Sunderland, E.M. et al., 2009. Mercury sources, distribution, and bioavailability in the North Pacific Ocean: Insights from data and models. Global Biogeochemical Cycles, 23(2), p.GB2010. | * Sunderland, E.M. et al., 2009. Mercury sources, distribution, and bioavailability in the North Pacific Ocean: Insights from data and models. Global Biogeochemical Cycles, 23(2), p.GB2010. | ||
| − | * Van Gilder Cooke, S., 2011. Coral “whisperers” diagnose reef pollution woes - environment - 24 February 2011 - New Scientist. newscientist.com | + | * Van Gilder Cooke, S., 2011. Coral “whisperers” diagnose reef pollution woes - environment - 24 February 2011 - New Scientist. newscientist.com. |
* Venn, A.A. et al., 2009. P-glycoprotein (multi-xenobiotic resistance) and heat shock protein gene expression in the reef coral Montastraea franksi in response to environmental toxicants. Aquatic Toxicology, 93(4), pp.188–195. | * Venn, A.A. et al., 2009. P-glycoprotein (multi-xenobiotic resistance) and heat shock protein gene expression in the reef coral Montastraea franksi in response to environmental toxicants. Aquatic Toxicology, 93(4), pp.188–195. | ||
* Victor, S. & Richmond, R.H., 2005. Effect of copper on fertilization success in the reef coral Acropora surculosa. Marine pollution bulletin, 50(11), pp.1448–1451. | * Victor, S. & Richmond, R.H., 2005. Effect of copper on fertilization success in the reef coral Acropora surculosa. Marine pollution bulletin, 50(11), pp.1448–1451. | ||
| Line 206: | Line 209: | ||
* Wooldridge, S.A., 2010. Is the coral-algae symbiosis really “mutually beneficial” for the partners? BioEssays, 32(7), pp.615–625. | * Wooldridge, S.A., 2010. Is the coral-algae symbiosis really “mutually beneficial” for the partners? BioEssays, 32(7), pp.615–625. | ||
* Work, T.M., Aeby, G.S. & Maragos, J.E., 2008. Phase Shift from a Coral to a Corallimorph-Dominated Reef Associated with a Shipwreck on Palmyra Atoll C. R. McClain, ed. PLoS ONE, 3(8), p.e2989. | * Work, T.M., Aeby, G.S. & Maragos, J.E., 2008. Phase Shift from a Coral to a Corallimorph-Dominated Reef Associated with a Shipwreck on Palmyra Atoll C. R. McClain, ed. PLoS ONE, 3(8), p.e2989. | ||
| − | + | ||
| + | Written by Kaho H. Tisthammer (kahot@hawaii.edu), Kewalo Marine Laboratory, University of Hawaii at Manoa | ||
| + | |||
| + | |||
| + | |||
| + | {{author | ||
| + | |AuthorID=31301 | ||
| + | |AuthorFullName=Kaho H. Tisthammer | ||
| + | |AuthorName=Arielmika}} | ||
| + | |||
| + | |||
| + | [[Category:Coastal and marine pollution]] | ||
| + | [[Category:Ecotoxicology]] | ||
| + | [[Category:Coral reefs]] | ||
Latest revision as of 11:42, 14 September 2020
Contents
An Overview of the Ecotoxicology of Coral
Abstract
Coral reefs worldwide are undergoing serious threats from anthropogenic pressures. Traditionally, coral reef assessment and monitoring programs use mortality, such as percent death or species loss, as an indicator, which is not an effective metric for heath. Coral ecotoxicology has emerged to apply biomedical techniques to ‘diagnose’ coral heath at a sublethal level. In order to protect coral reefs from further damages, it is critical to detect biological stress responses at the early stages so that the process may be reversed. Increasing numbers of coral ecotoxicological studies have shown the effective use of such techniques in the past decade. This review provides overview of molecular biomarkers used in coral ecotoxicological studies to detect sublethal responses in reef-building, scleractinian coral. We summarize the major cellular stress-response pathways characterized in corals, as well as the effects of major stress-inducing agents on coral are discussed.
I. Introduction
Coral reefs worldwide are in deep trouble due to negative impacts from human activities and climate change (Wilkinson, 2004; Hughes et al., 2010; Richmond & Wolanski, 2011; Graham, 2014). In order to assess the health of coral reefs, a number of ecological monitoring programs have been developed. However, most of the current coral reef assessment and monitoring programs still largely use mortality as an indicator (e.g. loss of species and coral cover reductions), which is an “ineffective metric of stress when the desired outcome is health” (Richmond & Wolanski 2011). Traditional reef monitoring and assessment cannot determine the exact causes, as well as the relative contribution of multiple stressors (Downs et al. 2005). In order to assess the health of coral reefs more effectively (i.e. identifying the stressors, figuring out their causative effects, and detecting stress early), researchers began to use biomedical research tools to diagnose environmental health, including corals (Richmond & Wolanski 2011; Downs et al. 2005). This gave a rise to coral ecotoxicology, a relatively ‘young’ field, which studies the effects of chemicals and potential pollutants on individual organisms with the intent of applying such data to understanding their effects on populations and community structures and functions (Richmond 2004). Ecotoxicology aims to predict the effects of potential pollutants at the population, community or ecosystem level so that we can prevent or remediate possible detrimental situations effectively and efficiently. Coral ecotoxicology is designed to ‘diagnose’ coral health at a sublethal level, since stress response, such as changes in protein production and gene expression, usually occurs before physiological damage is evident (Rougée 2011; Downs et al. 2005). Coral ecotoxicological studies investigate various physiological responses to stress-inducing agents, such as protein production related to detoxification and oxidative damage, changes in growth rate, larval settlement, and bleaching (Downs et al. 2012; Ferrier-Pagès et al. 2001; Fitt et al. 2001; Negri & Heyward 2001). A number of biomarkers, or biological indicators, have been identified and used to assess the stress responses in coral. Biomarkers can be categorized into two main groups, molecular and non-molecular. The molecular markers assess changes in protein expression levels, enzyme activity levels, DNA damage, and gene expression levels. The non-molecular markers include measuring the effects on fertilization success, larval-settlement, metamorphosis, growth rates and mortality/survivorship. This article provides a comprehensive overview of the coral ecotoxicological studies conducted recently in both the laboratory and the field. First, molecular biomarkers used in coral ecotoxicological studies are summarized along with the major cellular stress-response pathways characterized in coral. Second, by shifting the focus from biomarkers to stress-inducing agents, the effects of major inducing agents on coral are summarized. Lastly, an example of a successful study utilizing the ecotoxicology technique is introduced with the summary.
II. Cellular/sub-cellular Stress Responses in Coral (Molecular Biomarkers)
A number of laboratory exposure experiments and field studies have been conducted to assess the responses to stress-inducing agents in coral using molecular and non-molecular biomarkers. The focus of this review is on molecular markers, which are often employed to detect sublethal, cellular-level responses to stress-inducing agents.
1) Protein Expression / Enzyme Activity Levels:
Changes in certain protein production or enzymatic activity have been employed as molecular markers in coral ecotoxicological studies in order to detect the sublethal stress response. Several major stress response pathways are fairly well understood in coral, and summarized below, along with the molecular biomarkers associated with these pathways.
Xenobiotic and detoxification response
Xenobiotic metabolism is characterized in a number of species of coral (Rotchell & Ostrander 2011). Coral cells contain detoxification enzymes that metabolize and expel harmful xenobiotics by altering their chemical structure, making them less toxic and more easily excreted. This generally involves a 3-phase process (Figure 1). The Phase I enzymes catalyze reactions such as hydrolysis, reduction, and oxidation of xenobiotics, and make them slightly more hydrophilic (Jokanović 2001). Cytochrome P450 monooxygenase (CYP), a large family of enzymes with broad substrate specificity, have been used to measure this Phase I activity (e.g. Downs et al. 2012; Downs et al. 2006; Rougée et al. 2006; Rotchell & Ostrander 2011). In Phase II, the conjugative reactions add the Phase I metabolites with endogenous substrates such as sulfates, acetates, glutathione, and glucuronic acid, which increase the toxicant’s water solubility and facilitate excretion (Downs, et al. 2012). Glutathione-S-transferase (GST), which conjugates glutathione to toxic metabolites, is used to measure the Phase II activity in coral (e.g. Downs et al. 2012; Downs & Downs 2006; Rougée et al. 2006). The last stage involves the transportation of the hydrophilic metabolites to lysosomes for further metabolism for containment or excretion from the cell. The biomarker for this process is multixenobiotic resistance protein (MXR). MXR is an ATP-binding cassette transporter that exports glutathione-conjugated compounds out of the cell (e.g. Downs et al. 2012; Downs et al. 2006; Rougée et al. 2005). CYP, GST and MXR are the primary biomarkers to assess the xenobiotic response in coral. Most studies use all of these biomarkers to ensure the presence of the response (Downs et al. 2012; Downs et al. 2009; Downs & Downs 2006; Downs et al. 2006; Rougée et al. 2006). Table 1 summarizes the biomarkers that are used for assessing the xenobiotic response in coral.
Examples: Significantly elevated expression of CYP (6-class) was reported in coral Pocillopora damicornis in Guam from a reef located near a golf course, compared to the reference site (Downs et al. 2012). This golf course site was known to experience elevated levels of sedimentation periodically due to run-off. Based on the elevated xenobiotic-response biomarker level, the corals at the golf course site were speculated not only experiencing sedimentation stress, but also responding to pesticide exposure from golf course run-off (Downs et al. 2012). The significantly increased xenobiotic-response biomarker , GST activity, was induced in Montastraea faveolata by exposure to high concentrations of Benzo[a]pyrene (BaP), a component of various fuels and oils (Ramos & García 2007). Significantly higher GST and MXR were also reported in P. damicornis following laboratory exposures to crude oil (Rougée et al. 2006). These results elucidate that corals possess the detoxification mechanism for petrogenic substances such as oils and fuels.
Oxidative damage and stress biomarkers
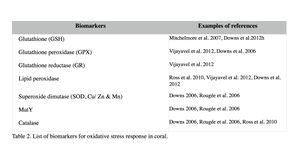
Oxidative damage/stress results from the production of reactive oxygen species such as free radicals and peroxide, and can be induced in coral by a variety of stressors, including toxic chemicals, abnormal temperatures, and UV irradiation (Rotchell & Ostrander 2011). This oxidative stress can damage lipids, proteins and DNA, and may lead to cell death if the stress continues (Downs et al. 2002). Oxidative stress response can be measured by the changes in antioxidant production or enzymatic activity level. Biomarkers used to assess oxidative stresses in coral are summarized in Table 2 (e.g. Downs et al. 2012; Vijayavel et al. 2012; Downs et al. 2011; Rotchell & Ostrander 2011; Rougée et al. 2006; Downs et al. 2002). Cells respond to the production of damaging oxygen radicals by producing antioxidant enzymes and proteins to neutralize these reactive oxygen species (Figure 2) (Rotchell & Ostrander 2011). For example, glutathione (GSH) is one of the major antioxidants in cells, and the altered GSH levels can be measured by the kinetic assay of glutathione peroxidase (GPX), glutathione reductase (GR), or the GSH content directly or indirectly (e.g. using free thiols and proteic thiol in Farina et al. 2008).
Examples: Following exposure to BaP, coral larvae of Porites astreoides showed a significant increase in GSH content (Farina et al. 2008), indicating that BaP induced oxidative stress, in addition to the xenobiotic response in coral as previously described. Excessive concentrations of iron are also known to lead to the generation of free radical species, resulting in the oxidative stress and peroxidation of membrane lipids in cells (Vijayavel et al. 2012). Oxidative stress induced by iron in larvae of P. damicornis is discussed further in Section III. Coral larvae of P. astreoides showed the oxidative stress response to a toxin produced by the dinoflagellate, Karenia brevis. K. brevis produce a series of neurotoxins and cause red tides in Florida (Ross et al. 2010). Following exposure to live cells or cellular lysates of K. brevis, the activity levels of lipid hydroperoxide and catalase, as well as concentrations of carbonyl in P. astreoides were significantly elevated, indicating oxidative stress. Although no significant reduction was observed in larval settlement and survival in this experiment (after 20-hour exposure), Ross et al. (2010) state that these coral larvae are unlikely to survive a longer exposure to the toxin of K. brevis due to the high-level of oxidative injury observed.
Protein metabolic condition
Changes in certain protein production and turnover indicate a significant shift in cellular metabolism and homeostasis (Rougée et al. 2006; Downs et al. 2006). The biomarkers for protein metabolism are listed in Table 3. Heat shock proteins (Hsp) are a chaperone that ensures the correct protein foldings, and binds to denatured proteins to prevent them from causing cell damage (Rougée et al. 2006). They are called ‘stress proteins’ since their expression is elevated by not only thermal stress but also a variety of other stressors, such as heavy metals, UV, and toxic chemicals (Santoro 2000). Elevated levels of these proteins also indicate a significant system-wide shift in the protein metabolic conditions and equilibrium (Downs et al. 2012; Downs et al. 2006; Rougée et al. 2006). Ubiquitin and ubiquitin ligase are involved in the degradation of broken or unnecessary proteins. Their up-regulation indicates that a demand for protein degradation has increased, or the process has amplified (Downs & Downs 2006; Downs et al. 2006).
Examples: Upregulation of heat shock proteins in coral was reported from a number of studies. For example, field studies conducted to assess the general health of coral reefs in the Virgin Islands (P. astreoides) and Guam (P. damicornis) report that high heat shock protein levels, along with other biomarkers, were detected from potentially polluted coral reef sites (Downs et al. 2012; Downs et al. 2011).Theses sites had higher concentrations of pesticides, herbicides and fungicides than the reference sites, indicating the overall stress levels of these reefs were higher. Gene expressions are also used to detect changes in the heat shock protein expression in coral (see II-3 for more information). For instance, increased expression levels of the Hsp 70 genes were reported after exposing Montastraea franksi to copper and an oil dispersant (Corexit 9527), and Hsp 90 gene expression was also elevated by oil dispersant exposure (Venn 2009).
Others
Other characterized stress response pathways in coral include porphyrin metabolism and homeostatic changes. These pathways are shown to be altered/disrupted by exposure to a variety of stressors. The biomarkers used to assess porphyrin metabolism (Table 4) and metabolic homeostasis (Table 5) are summarized below.
2) DNA damage
Several studies have reported DNA damage in coral from exposure to stress-inducing agents, using different techniques. The standard COMET assay (single cell gel electrophoresis) was successfully applied to quantify the increases in DNA damage after exposing Montastraea franksi to copper of different dilutions (1). Alternatively, the DNA damage can be assessed by measuring the formation of DNA adducts, which are covalent adducts formed between chemical mutagens and DNA. The DNA adducts-formation was reported from P. lobata exposed to hydrocarbon after the oil-spill incident in Yap (Downs et al. 2006). In addition, increased apurinic/apyrimidinic (AP) sites, which are DNA without a purine or a pyrimidine base due to the damage/stress, were observed as a response to exposure of P. damicornis larvae to iron chloride (Vijayavel et al. 2012). The field study sites in the Virgin Islands also showed higher AP sites in P. astreoides, where high concentrations of pollutants were detected (Downs et al. 2011). If we include environmental stressors, several more studies have reported DNA damage in coral. Physical damage to DNA was observed after exposing the cell suspensions of Stylophora pistillata to UVB irradiation (Baruch 2005). Increased AP sites were also observed by exposing S. pistillata to hyposalinity (28 ppt, 24 ppt, 20 ppt) (Downs et al. 2009). Additionally, an elevated level of MutY enzyme, the DNA glycosylase that repairs oxidative damage to DNA, may indicate the DNA damage is occurring in the cell (Downs et al. 2006).
3) Gene expression
Increasing numbers of studies have demonstrated that gene expression can evaluate the relative impact of stressors in coral. Upregulation or downregulation of gene expressions associated with stress-response pathways are reported in several studies (e.g. Edge et al. 2005). The coral genes studied as stress response to inducing agents include:
- Heat shock protein (Hsp70, Hsp90) genes - the expression was upregulated by thermal stress, Cu exposure, & oil dispersant exposure (Császár et al. 2009; Venn et al. 2009).
- Ferritin gene - the expression was upregulated by thermal stress (Császár et al. 2009).
- Superoxide dimutase (SOD-Mn) gene - the expression was upregulated by thermal stress (Császár et al., 2009).
- Zn2+-metalloprotease gene - the expression was upregulated by thermal stress (Császár et al., 2009).
- Carbonic anhydrase gene - the expression was downregulated by thermal stress, but upregulated by hypersalinity & UV (Edge et al. 2005).
- uPAR (urokinase plasminogen activator receptor) gene - the expression was upregulated by hypersalinity & UV, but the mixed response was obtained by thermal stress (Edge et al. 2005).
- P-glycoprotein (P-gp)(multi-xenobiotic resistance protein) gene - upregulated by Cu and oil dispersant exposure (Venn et al. 2009).
- Metallothionein coding gene - (this gene is known to be involved in stress response, and was successfully isolated from Acropora cervicornis, but the expression was not induced by thermal, UV or hypersalinity stress [Edge et al. 2005; Snell et al. 2003]).
Measuring stress responses using gene expressions in coral is a rapidly developing filed and will require further studies to understand the mechanism comprehensively. With an advancement of next generation sequencing (NGS) techniques, researchers now can capture the whole transcriptomic response to toxicants or other environmental stressors without specific knowledge on the mechanisms or pathways involved. However, the relationship between stress responses and gene expressions can be complex, as can be seen in the unique gene expression profiles observed in Edge et al. (2005)’s study, which showed uPAR -urokinase plasminogen activator receptor- upregulated the most at the intermediate stress level. Nevertheless, this ‘toxicogenomic’ approach has a great potential to diagnose sublethal stress in coral and advance our knowledge.
4) Other endpoints
The effects of stress-inducing agents in coral can be assessed by a variety of endpoints other than molecular biomarkers, such as fertilization success, larval settlement, metamorphosis, growth rates, and mortality/survivorship. Although non-molecular biomarkers are not the focus of this review, the major ecotoxicological responses measured by non-molecular markers are summarized in Table 7 (some of these responses will be discussed in the next section). Early life stages of many marine organisms are found to be more sensitive to pollutants than adult stages (Reichelt-Brushett & Harrison 1999). This is probably due in part to the important role that chemical cues play in substrate selection and metamorphosis of larvae in many marine organisms (Hadfield & Paul 2001). Indeed, select species of coral larvae use chemical cues to settle on a substratum where a specific coralline algal species is present (Golbuu & Richmond 2007). Therefore, it is critical to understand the effects of pollutants on gametes and larvae.
III. Responses of coral to specific stressors:
Relatively high numbers of laboratory coral exposure experiments to various stress-inducing agents are reported. However, the number of field studies is rather limited, although the results have been informative. The following section summarizes the effects of major stress-inducing agents (heavy metals and organic compounds) in coral.
1. Heavy Metals
Copper (Cu):
Copper (Cu) is an essential element to all life, but can be toxic to some marine organisms, including coral, even at relatively low concentrations (Victor & Richmond 2005). Cu occurs naturally in the marine environment. Its concentrations in seawater are reported to be 0.01 - 0.03 μg/l in relatively pristine marine environments (Victor & Richmond, 2005), but it could range up to 50 μg/l at highly polluted sites (Mitchelmore et al. 2007; Reichelt-Brushett & Harrison 1999). Anthropogenic sources of Cu are diverse: Cu is found in antifouling paints, sewer discharge, fungicides, herbicides, construction materials of waterfront structures, and heat exchangers in power plants (Victor & Richmond 2005). In adult coral, Cu is reported to accumulate in tissues (and the symbiont algal tissues), and Cu exposure induces oxidative stress response. Even exposure at low Cu concentrations (≦5 μg/l) of P. damicornis in laboratory experiments resulted in a significantly elevated level of GSH (Mitchelmore et al. 2007). A 14-day 50 μg/l of Cu exposure resulted in 100% mortality of P. damicornis (Mitchelmore et al. 2007). Furthermore, Bielmyer et al. (2010) reported significant decreases in skeletal growth in A. cervicornis and P. damicornis. Interestingly, the relationships between physiological/toxicological endpoints and Cu accumulation differed among 3 coral species they studied, which indicates the presence of interspecific differences in sensitivity to Cu. Cu is known to affect not only coral but also its symbiotic algae, and the reduction in photosynthetic levels, as well as bleaching, has been reported (Negri & Hoogenboom 2011). However, one experiment shows that Cu induced coral bleaching without affecting the algal photosynthesis, thereby confirming that Cu directly affects corals (Jones 2004). Bielmyer et al. (2010) also reported that no changes in zooxanthellae communities were apparent in response to increasing Cu concentrations. Coral larvae and juveniles show high sensitivity to Cu. Laboratory experiments show that Cu inhibits fertilization success (Victor & Richmond 2005; Reichelt-Brushett & Harrison 1999), survival of gametes (Victor & Richmond 2005), metamorphosis of larvae (Negri & Hoogenboom 2011), and larval settlement & survival (Reichelt-Brushett & Harrison 2000). According to Reichelt-Brushett & Harrison (2000), Cu inhibits the metamorphosis of coral larvae at lower concentrations than any metal tested so far.
Iron (Fe):
Iron (Fe) is an important limiting nutrient in the marine ecosystem, especially in the Central Pacific Ocean (Butler 1998). Iron concentrations are extremely low in seawater, normally less than 1 nmol /l (Spokes & Croot 2003). Elevated Fe levels, thus, could dramatically enhance primary production, causing algal blooms and community structural changes (phase shifts) in coral reefs (Kelly et al. 2012; Work et al. 2008; Martin & Fitzwater 1988). Excessive concentrations of iron will also lead to reactive oxygen species generation (Figure 3). The sources of Fe that cause unusually high Fe concentrations include ship groundings and discharges from iron-ore mining, smelters and steel plants (Kelly et al. 2012; Vijayavel et al. 2012). Ali et al. (2010) found a significant correlation between seawater iron concentrations and dead coral cover in the Red Sea, suggesting chronic exposure to higher Fe levels may reduce coral growth rates. In a laboratory exposure experiment, Vijayavel et al. (2012) investigated sublethal effects of dissolved Fe on larvae of P. damicornis. They found that the high iron chloride concentration (100ppm) induced oxidative stress response, including significantly increased activity levels of lipid peroxidase, GR, and GST, and decreased levels of GSH and GPX activity. Additionally, DNA damage (AP site formation) was reported at 100 ppm of Fe (as previously mentioned in Section II-3) (Vijayavel et al. 2012).
Cadmium (Cd):
The effects of Cadmium (Cd) have been studied in several laboratory exposure experiments. Unlike Cu and Fe, Cd does not have an essential biological function, and is known to cause oxidative stress and alter the calcium homeostasis in coral (Mitchelmore et al. 2007). Cd concentrations in seawater are normally less than 3μg/l, but concentrations up to 50 μg/l have been reported in polluted harbors and ports (Mitchelmore et al. 2007; Reichelt-Brushett & Harrison 1999). More than 90% of Cd in the marine environment is known to be of anthropogenic origin. The run-off /discharge from the smelting of metal ores is estimated to be the largest Cd source (Ali et al. 2010; Nriagu & Pacyna, 1988). Mitchelmore et al. (2007) reported that exposure of adult P. damicornis to 5 μg/l of Cd induced a significant increase in GSH, indicating an oxidative stress. In the same study, exposure of adult P. damicornis to 50 μg/l of Cd lead to 100% mortality by day 4. Significant accumulation of Cd in coral tissues was also observed, though reduction in algal density was not reported in this study. On the other hand, Cd did not affect the fertilization success of Goniastrea aspera and Oxypora lacera in a laboratory exposure experiment where coral gametes were exposed to 200 - 1,000 μg/l of Cd (Reichelt-Brushett & Harrison 1999). This result was somewhat puzzling, for larvae/juveniles are generally more sensitive to stressors than adults, although marine organisms tend to show less sensitivity to Cd than to Cu (Bellas 2006; Reichelt-Brushett & Harrison 1999). At much higher concentrations of Cd (5,000 - 10,000 μg/l), which is unlikely to be seen in the open ocean, the fertilization success was significantly reduced in Acropora tenuis, but not in the other three coral species tested at the same time (Reichelt-Brushett & Harrison, 2005). The effects of Cd on coral seem to be complex, as the toxicity of Cd is known to be enhanced by increased temperature and/or decreased salinity of seawater (Reichelt-Brushett & Harrison 1999). Experimental designs that can address inter-specific differences as well as combinations of multiple stressors may help clarify the effects of Cd on gametes and larvae.
Mercury (Hg):
Mercury (Hg) is not essential to living cells and performs no known biological function, and Hg and most of its compounds are extremely toxic (Nichols 1997). The average concentration of Hg in ocean water is relatively low, ranging from 0.5 -12.5 ng/l (Rice et al. 1997). The major source of Hg in open oceans is recognized as atmospheric deposition, two-thirds of which is of anthropogenic origin, from mining and other industrial activities (Bastidas & García 2004). Other non-point Hg sources include erosion, runoff, flooding, overuse of agrochemicals, and industrial waste (Guzmán & García 2002). Recently Sunderland et al. (2009) raised a concern that the total Hg concentration in the oceans is increasing. Their modeling results suggest that basin-wide Hg concentrations will double by 2050 from 1995 values. Hg accumulates in coral tissues, as well as coral skeletons (Markey et al. 2007; Guzmán & García 2002). The effects of Hg in coral have not been studied extensively, but the sublethal effects have been reported by Farina et al. (2008) and Bastidas & García (2004). Exposing adult P. astreoides to Hg concentrations of 0.04 - 0.249 mg/l resulted in physiological deterioration, such as polyp color darkening and colony tissues being peeled off by excess mucous sheets (Bastidas & García 2004). Significant decreases in polyp biomass, zooxanthellae density/biomass, and pigment concentration were also reported. Exposure at the highest Hg concentration of 0.249 mg/l resulted in polyp contraction at 8 hours, color and tissue loss at 24-48 hours, and 50% mortality in 72 hours, suggesting Hg concentrations between 0.037 and 0.180 mg/l to be lethal (LC50–72 h) for P. astreoides (Bastidas & García 2004). The effects of Hg on larvae were studied by exposing P. astreoides larvae to 10 μg/l of Hg for 96 hours. Interestingly, the experiment did not affect the survivorship of the larvae compared to the control (Farina et al. 2008). Catalase and GST activities were also unaltered in this exposure experiment, although the GST activity level in P. astreoides larvae was found to be 3.5 times higher than in the adults.
Zinc (Zn):
Zinc is an essential element for all life, and Zn requirements for organisms are usually higher than for Cu (Reichelt-Brushett & Harrison 2004). The average Zn concentration recorded in the world’s oceans is approximately 5 μg/l, although much higher amounts are recorded in sewage and industrial waste discharge (Reichelt-Brushett & Harrison 1999). The results from a laboratory exposure experiment of G. aspera gametes to Zn did not show any significant reduction in fertilization success up to 500 μg/l of Zn concentrations (Reichelt-Brushett & Harrison 1999). However, a reduction in the fertilization success of Favites chinensis and Platygyra ryukyuensis was previously reported by Heyward (1988) at 500-1000 μg/l of Zn. According to Reichelt-Brushett & Harrison (1999), the concentrations of Zn used in their study were relatively low and the exposure time was short (5-hour), suggesting that exposure at higher concentrations or for longer periods of time may be necessary to detect the effects. Additionally, there seem to exist interspecific differences in sensitivities not only to Zn, but also to Cu and Cd. Therefore, generalizing from one species to another may be difficult for certain stressors (Reichelt-Brushett & Harrison 1999; Heyward 1988). Further studies are needed to conclude the effects of Zn on coral fertilization, and to find out the interspecific differences in sensitivity to Zn.
Metal accumulation in coral as a pollution indicator:
Although accumulation of Cd in coral shows somewhat variable results (Mitchelmore et al. 2007), it appears that most metal concentrations in coral skeletons demonstrate a positive correlation between the metal concentrations in seawater and sediment. Therefore, skeletal concentrations could serve as a pollution level indicator (Bin Mokhtar et al. 2012; Inoue 2004). Zn, Mn, Ni, Fe, Cd, and Cu show, in this order, high bioaccumulation factors in coral skeletons (Bin Mokhtar et al. 2012). Metal accumulations in coral tissues, however, vary possibly due to the differences in uptake capacity, excretion, and detoxification ability (Ali et al. 2010), and therefore may not serve as a good indicator of pollutants.
2. Organic Compounds
The effects of organic compounds such as insecticides, anti-fouling agents and hydrocarbons have been studied in coral as the nearshore water quality continues to deteriorate and threaten coral reefs. Major sources of organic pollutants are coastal development, increased run-offs, and toxic antifouling paints from ships (Knutson et al. 2011; Negri & Marshall 2008; Negri et al. 2002; Negri & Heyward 2001).
TBT:
Due to their toxicity, antifouling paints that contain organotin compounds, such as tributylin (TBT), have been banned by the International Maritime Organization (IMO). Applying or reapplying paints with TBT has been banned since 2003, yet as of 2008 only 34 out of 168 nations have ratified the Convention on the Control of Harmful Anti-fouling Systems on Ships (International Maritime Organization 2012). Therefore, TBT still poses a threat to marine organisms. Furthermore, this compound is stored in sediments and re-enters the food chain when the sea bottom is stirred up (Adamson 2003). Effects of organotin (TBT) are relatively well studied in coral, and have been shown to affect their entire life history, such as reduction in fertilization, inhibition of larval settlement and metamorphosis, and bleaching in juveniles as well as in adults (Negri & Marshall 2009; Inoue et al. 2004; Negri et al. 2002).
Irgarol:
Since the banning of TBT in 2003, the use of Irgarol 1051, an algaecide added to marine antifouling paints, has increased dramatically (Knutson et al. 2011). Irgarol inhibits the photosynthesis II pathway, and thus the negative effects of Irgarol in coral that harbor symbiotic zooxanthellae have been reported in the coral species Galaxea fascicularis, Seriatopora hystrix, and Madracis mirabilis (Knutson et al. 2011; Downs & Downs 2006). For example, exposure of M. mirabilis to 10 μg/l of Irgarol induced oxidative stress and xenobiotic responses in both coral and zooxanthellae, as well as porphyrin synthesis disruption in coral (Downs & Downs 2006). Irgarol also has inhibitory effects on coral larval settlement, raising a serious concern as larvae seem to be highly sensitive to Irgarol. Concentrations of Irgarol ≧100 ng/l caused a significant decrease in the settlement of Porites hawaiiensis larvae (Knutson et al. 2011). According to Knutson et al. (2011), typical aqueous concentrations of Irgarol in marinas and tropical coastal seawaters are below 200 ng/l. Irgarol concentrations in various marinas in Oahu, Hawaii ranged from <17 - 283 ng/l. Even normal use of Irgarol, therefore, could inhibit larval settlement in coral, and the use of Irgarol may need to be reevaluated.
Hydrocarbons:
Exposure of coral to hydrocarbons, such as fuel oils, has been evaluated in several studies. Downs et al. (2006) conducted a field study to assess the cellular-level effects of fuel oil exposure from ship groundings in Yap. The ecotoxicological assessment of P. lobata showed a clear indication of xenobiotic response, oxidative stress response, disrupted porphyrin and protein metabolism. Additionally, BPDE adducted protein lesions were detected in coral, confirming exposure of corals to polycyclic aromatic hydrocarbons. The highly carcinogenic BPDE is a metabolite of BaP, and can readily adduct to DNA, RNA, and protein (Rotchell & Ostrander 2011). Additionally, several laboratory exposure experiments were conducted to assess the effects of BaP, crude oil, and oil dispersant. Laboratory experiments exposing P. damicornis to crude oil induced significant xenobiotic response, oxidative stress response, and protein & porphyrin metabolism disruption (Rougée et al. 2006), as did the field study conducted by Downs et al. (2006). Exposure of P. astreoides larvae to 10 μg/l of BaP induced oxidative stress response (increased GSH concentrations) and significantly reduced survivorship (Farina et al. 2008). However, catalase activity was unaltered in this experiment. Adult M. franksi exposed to the oil dispersant, Corexit 9527, also induced a stress response, which was measured by an increase in the gene expression of P-gp and Hsp70 (Venn et al. 2009).
Summary
Ecotoxicology is a diagnostic tool used to assess coral reef health at a sublethal level, and the technique has proven successful, as in the oil-spill study in Yap. Following the grounding of the Kyowa Violet vessel in Yap in 2002, Downs et al. (2006) conducted a field study to assess damage in coral in areas not visibly oiled. They used molecular biomarkers to assess the sublethal cellular stress levels in coral by comparing the oil-spill site with two reference sites. The results clearly demonstrated elevated levels of physiological stress in corals at the oil-spill site: oxidative stress response, xenobiotic response, changes in protein metabolism conditions and porphyrin metabolism were all significantly different from the reference sites. The results were also analyzed by canonical correlation analyses using multiple biomarkers, visualized in Figures 4 - 6. Figure 4 indicates that the xenobiotic response (CYP) profiles of corals at Ruul Causeway, the oil-spill site, were significantly different from those in Tamil and South Ruul, the 2 reference sites. Figure 5 shows that Ruul Causeway corals had a significant oxidative stress condition compared to the reference sites. Figure 6 indicates that overall, the cellular physiological state of the corals at Ruul Causeway was significantly altered from those of the reference sites. Also, in order to determine the causative nature, an additional molecular biomarker was used specifically for this study to assess the effects of BaP. As mentioned in the previous section on Hydrocarbons, the presence of the BPDE adducted proteins provided the evidence of exposure to oils. Due partly to the evidence from this study, the Yap islanders were awarded $861,600 for the damage to the reef (Van Gilder Cooke 2011). This example also signifies the fact that, for coral reef conservation, the results of ecotoxicological studies that show clear causative effects of toxicants are highly useful for resource managers to make decisions on toxic pollutants and chemicals.
As a relatively young field, coral ecotoxicology has numerous unanswered questions. Differences in sensitivities to stressors were observed among coral species (as mentioned in Section II). However, as most studies use a single species for laboratory experiments or field studies, the variability in stress responses among different species are largely unknown. Further studies are required to better understand the varied responses among species. The response variability among individuals, as well as among populations, is also unknown since laboratory experiments normally use coral samples from a single colony (in order to reduce the individual variability). Field studies have collected samples from different individuals, yet the number of samples has been too small (n=6~10) to resolve the variability at the individual level. The relationship between the interspecific (and intraspecific) variability of stress responses and the associated genetic variability responsible for the differences is an interesting topic to be explored, and may also contribute to the conservation of coral reefs in the future. With dramatic progress in molecular techniques, increasing amounts of genetic and genomic information have become available in the coral ecotoxicology field. Assessing the relationships between protein expression and gene expression is now possible, which would further strengthen the results, as well as decode some of the complex stress-responses observed in coral. This paper summarized the effects of stress-inducing agents on coral. However, the physiological state of coral’s symbiotic algae, zooxanthellae, is known to affect the cellular conditions in coral (Wooldridge 2010; Downs et al. 2002). Therefore, we need to incorporate the potential interactions between the stress responses of the host and the symbiont in order to fully understand ecotoxicological effects on coral. The field of coral ecotoxicology is expanding, and it is my hope that our research will help protect coral reefs and their biodiversity for future generations.
References:
- Adamson, L., 2003. Anti-fouling systems, London, UK: International Maritime Organization.
- Ali, A.-H.A.M., Hamed, M.A. & Abd El-Azim, H., 2010. Heavy metals distribution in the coral reef ecosystems of the Northern Red Sea. Helgoland Marine Research, 65(1), pp.67–80.
- Baruch, R., 2005. UV incites diverse levels of DNA breaks in different cellular compartments of a branching coral species. Journal of Experimental Biology, 208(5), pp.843–848.
- Bastidas, C. & García, E.M., 2004. Sublethal effects of mercury and its distribution in the coral Porites astreoides. Marine ecology progress series. Oldendorf, 267, pp.133–143.
- Bellas, J., 2006. Comparative toxicity of alternative antifouling biocides on embryos and larvae of marine invertebrates. Science of The Total Environment, 367(2-3), pp.573–585.
- Bielmyer, G.K. et al., 2010. Differential effects of copper on three species of scleractinian corals and their algal symbionts (Symbiodinium spp.). Aquatic Toxicology, 97(2), pp.125–133.
- Bin Mokhtar, M. et al., 2012. Trace metal (Cd, Cu, Fe, Mn, Ni and Zn) accumulation in Scleractinian corals: A record for Sabah, Borneo. Marine pollution bulletin, pp.1–8.
- Butler, A., 1998. Acquisition and utilization of transition metal ions by marine organisms. Science, 281(5374), pp.207–209.
- Császár, N., Seneca, F.O. & van Oppen, M., 2009. Variation in antioxidant gene expression in the scleractinian coral Acropora millepora under laboratory thermal stress. Marine Ecology Progress Series, 392, pp.93–102.
- Downs, C. & Downs, A., 2006. Preliminary Examination of Short-Term Cellular Toxicological Responses of the Coral Madracis mirabilis to Acute Irgarol 1051 Exposure. Archives of environmental contamination and toxicology, 52(1), pp.47–57.
- Downs, C.A. et al., 2011. A survey of environmental pollutants and cellular-stress markers of Porites astreoides at six sites in St. John, U.S. Virgin Islands. Ecotoxicology, 20(8), pp.1914–1931.
- Downs, C.A. et al., 2009. Cellular pathology and histopathology of hypo-salinity exposure on the coral Stylophora pistillata. Science of the Total Environment, The, 407(17), pp.4838–4851.
- Downs, C.A. et al., 2006. Cellular physiological effects of the MV Kyowa violet fuel-oil spill on the hard coral, Porites lobata. Environmental toxicology and chemistry / SETAC, 25(12), pp.3171–3180.
- Downs, C.A. et al., 2002. Oxidative stress and seasonal coral bleaching. Free radical biology & medicine, 33(4), pp.533–543.
- Downs, C.A. et al., 2005. Shifting the paradigm of coral-reef “health” assessment. Marine pollution bulletin, 51(5-7), pp.486–494.
- Downs, C.A. et al., 2012. The use of cellular diagnostics for identifying sub-lethal stress in reef corals. Ecotoxicology, 21(3), pp.768–782.
- Edge, S.E. et al., 2005. Development of a coral cDNA array to examine gene expression profiles in Montastraea faveolata exposed to environmental stress. Marine pollution bulletin, 51(5-7), pp.507–523.
- Farina, O. et al., 2008. Biochemical Responses of Cnidarian Larvae to Mercury and Benzo(a)pyrene Exposure. Bulletin of Environmental Contamination and Toxicology, 81(6), pp.553–557.
- Ferrier-Pagès, C. et al., 2001. Response of a scleractinian coral, Stylophora pistillata, to iron and nitrate enrichment. Journal of experimental marine biology and ecology, 259(2), pp.249–261.
- Fitt, W. et al., 2001. Coral bleaching: interpretation of thermal tolerance limits and thermal thresholds in tropical corals. Coral Reefs, 20(1), pp.51–65.
- Golbuu, Y. & Richmond, R.H., 2007. Substratum preferences in planula larvae of two species of scleractinian corals, Goniastrea retiformis and Stylaraea punctata. Marine Biology, 152(3), pp.639–644.
- Guzmán, H.M. & García, E.M., 2002. Mercury levels in coral reefs along the Caribbean coast of Central America. Marine pollution bulletin, 44(12), pp.1415–1420.
- Hadfield, M.G. & Paul, V.J., 2001. Chapter 13: Natural Chemical Cues for Settlement and Metamorphosis of Marine-Invertebrate Larvae. In J. B. McClintock & B. J. Baker, eds. Marine Chemical Ecology. Boca Raton, FL: CRC Press LLC, pp. 431–461.
- Heyward, A.J., 1988. Inhibitory effects of copper and zinc sulphates on fertilization in corals. Proceedings of the 6th Coral Reef Symposium, Australia, 2.
- Inoue, M., 2004. Coral skeletal tin and copper concentrations at Pohnpei, Micronesia: possible index for marine pollution by toxic anti-biofouling paints. Environmental Pollution, 129(3), pp.399–407.
- International Maritime Organization, 2012. IMO | International Convention on the Control of Harmful Anti-fouling Systems on Ships (AFS). imo.org. Available at: http://www.imo.org/about/conventions/listofconventions/pages/international-convention-on-the-control-of-harmful-anti-fouling-systems-on-ships-(afs).aspx [Accessed November 4, 2012].
- Jokanović, M., 2001. Biotransformation of organophosphorus compounds. Toxicology, 166(3), pp.139–160.
- Jones, R.J., 2004. Testing the “photoinhibition”model of coral bleaching using chemical inhibitors. Marine Ecology Progress Series, 284, pp.133–145.
- Kelly, L.W. et al., 2012. Black reefs: iron-induced phase shifts on coral reefs. The ISME journal, 6(3), pp.638–649.
- Knutson, S., Downs, C.A. & Richmond, R.H., 2011. Concentrations of Irgarol in selected marinas of Oahu, Hawaii and effects on settlement of coral larval. Ecotoxicology, 21(1), pp.1–8.
- Larson, A.M., 2007. Acetaminophen Hepatotoxicity. Clinics in Liver Disease, 11(3), pp.525–548.
- Martin, J.H. & Fitzwater, S.E., 1988. Iron deficiency limits phytoplankton growth in the north-east Pacific subarctic.
- Markey, K.L. et al., 2007. Insecticides and a fungicide affect multiple coral life stages. Marine Ecology Progress Series, 330, pp.127–137.
- Mitchelmore, C.L., Verde, E.A. & Weis, V.M., 2007. Uptake and partitioning of copper and cadmium in the coral Pocillopora damicornis. Aquatic Toxicology, 85(1), pp.48–56.
- Negri, A. & Marshall, P., 2009. TBT contamination of remote marine environments: Ship groundings and ice-breakers as sources of organotins in the Great Barrier Reef and Antarctica. Journal of Environmental Management, 90: S31–S40.
- Negri, A.P. & Heyward, A.J., 2001. Inhibition of coral fertilisation and larval metamorphosis by tributyltin and copper. Marine Environmental Research, 51(1), pp.17–27.
- Negri, A.P. & Heyward, A.J., 2000. Inhibition of fertilization and larval metamorphosis of the oral Acropora millepora by Petroleum Products. Marine pollution bulletin, 41(7), pp.420–427.
- Negri, A.P. & Hoogenboom, M.O., 2011. Water contamination reduces the tolerance of coral larvae to thermal stress. PLoS ONE, 6(5), p.e19703.
- Negri, A. et al. 2005. Effects of the herbicide diuron on the early life history stages of coral. Mar. Pollut. Bull. 51, pp. 370–383.
- Negri, A.P. et al., 2002. Understanding ship-grounding impacts on a coral reef: potential effects of anti-foulant paint contamination on coral recruitment. Marine pollution bulletin, 44(2), pp.111–117.
- Nichols, J.W., 1997. Mercury Study Report to Congress Volume VI, U.S. Environmental Protection Agency.
- Nriagu, J.O. & Pacyna, J.M. 1988. Quantitative assessment of worldwide contamination of air, water and soils by trace metals. Nature, 333: 134-139.
- Ramos, R. & García, E., 2007. Induction of mixed-function oxygenase system and antioxidant enzymes in the coral Montastraea faveolata on acute exposure to benzo(a)pyrene. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology, 144(4), pp.348–355.
- Reichelt-Brushett, A.J. & Harrison, P.L., 2004. Development of a sublethal test to determine the effects of copper and lead on scleractinian coral larvae. Archives of environmental contamination and toxicology, 47(1), pp.40–55.
- Reichelt-Brushett, A.J. & Harrison, P.L., 2000. The Effect of Copper on the Settlement Success of Larvae from the Scleractinian Coral Acropora tenuis. Marine pollution bulletin, 41(7), pp.385–391.
- Reichelt-Brushett, A.J. & Harrison, P.L., 1999. The effect of copper, zinc and cadmium on fertilization success of gametes from scleractinian reef corals. Marine pollution bulletin, 38(3), pp.182–187.
- Reichelt-Brushett, A.J. & Harrison, P.L., 2005. The effect of selected trace metals on the fertilization success of several scleractinian coral species. Coral Reefs, 24(4), pp.524–534.
- Rice, G.E. et al., 1997. Mercury study report to Congress. Volume 3. Fate and Transport of Meucury in the Environment, Environmental Protection Agency, Research Triangle Park, NC (United States). Office of Air Quality Planning and Standards.
- Richmond, R.H., 2004. Coral reproduction and recruitment as tools for studying the ecotoxicology of coral reef ecosystems. In Techniques in Aquatic Toxicology. pp. 1–10.
- Richmond, R.H. & Wolanski, E., 2011. Coral research past efforts and future horizons. In Z. Dubinsky & N. Stambler, eds. CORAL REEFS: AN ECOSYSTEM IN TRANSITION. Springer Science, pp. 3–12.
- Ross, C. et al., 2010. Effects of the Florida red tide dinoflagellate, Karenia brevis, on oxidative stress and metamorphosis of larvae of the coral Porites astreoides. Harmful Algae, 9(2), pp.173–179.
- Rotchell, J.M. & Ostrander, G.K., 2011. Molecular toxicology of corals: a review. Journal of toxicology and environmental health. Part B, Critical reviews, 14(8), pp.571–592.
- Rougée, L., 2011. Expression and Activity of Xenobiotic Metabolizing Enzymes in the Reef Coral Pocillopora damicornis.
- Rougée, L. et al., 2006. Alteration of normal cellular profiles in the scleractinian coral (Pocillopora damicornis) following laboratory exposure to fuel oil. Environmental toxicology and chemistry, 25(12), pp.3181–3187.
- Runnalls, L.A. & Coleman, M.L., 2003. Record of natural and anthropogenic changes in reef environments (Barbados West Indies) using laser ablation ICP-MS and sclerochronology on coral cores. Coral Reefs, 22(4), pp.416–426.
- Santoro, M.G., 2000. Heat shock factors and the control of the stress response. Biochemical pharmacology, 59(1), pp.55–63.
- Sastre, J., Pallardó, F.V. & Viña, J., 1996. Glutathione, oxidative stress and aging. Age, 19 (4), pp.129-139.
- Schwarz, J. A., et al. 2013. Comparative Biochemistry and Physiology, Part C. Comparative Biochemistry and Physiology, Part C 157, pp.272–279.
- Shafir, S., Van Rijn, J. & Rinkevich, B., 2007. Short and Long Term Toxicity of Crude Oil and Oil Dispersants to Two Representative Coral Species. Environmental Science & Technology, 41(15), pp.5571–5574.
- Snell, T.W., Brogdon, S.E. & Morgan, M.B., 2003. Gene expression profiling in ecotoxicology. Ecotoxicology, 12(6), pp.475–483.
- Spokes, L. & Croot, P., 2003. Iron in the oceans. Environmental Science Published for Everybody Roudn the Earth. Available at: http://www.atmosphere.mpg.de/enid/1vv.html [Accessed November 6, 2012].
- Sunderland, E.M. et al., 2009. Mercury sources, distribution, and bioavailability in the North Pacific Ocean: Insights from data and models. Global Biogeochemical Cycles, 23(2), p.GB2010.
- Van Gilder Cooke, S., 2011. Coral “whisperers” diagnose reef pollution woes - environment - 24 February 2011 - New Scientist. newscientist.com.
- Venn, A.A. et al., 2009. P-glycoprotein (multi-xenobiotic resistance) and heat shock protein gene expression in the reef coral Montastraea franksi in response to environmental toxicants. Aquatic Toxicology, 93(4), pp.188–195.
- Victor, S. & Richmond, R.H., 2005. Effect of copper on fertilization success in the reef coral Acropora surculosa. Marine pollution bulletin, 50(11), pp.1448–1451.
- Vijayavel, K. et al., 2012. Oxidative DNA damage induced by iron chloride in the larvae of the lace coral Pocillopora damicornis. Comparative Biochemistry and Physiology, Part C, 155(2), pp.275–280.
- Wakamatsu, T.H., Dogru, M. & Tsubota, K., 2008. Tearful relations: oxidative stress, inflammation and eye diseases. Arquivos brasileiros de oftalmologia, 71(6 Suppl), pp.72–79.
- Wilkinson, C., 2004. Status of coral reefs of the World: 2004. Volume 1. Status of coral reefs of the World.
- Wooldridge, S.A., 2010. Is the coral-algae symbiosis really “mutually beneficial” for the partners? BioEssays, 32(7), pp.615–625.
- Work, T.M., Aeby, G.S. & Maragos, J.E., 2008. Phase Shift from a Coral to a Corallimorph-Dominated Reef Associated with a Shipwreck on Palmyra Atoll C. R. McClain, ed. PLoS ONE, 3(8), p.e2989.
Written by Kaho H. Tisthammer (kahot@hawaii.edu), Kewalo Marine Laboratory, University of Hawaii at Manoa
Please note that others may also have edited the contents of this article.
|