Difference between revisions of "Polychlorinated naphthalenes"
Dronkers J (talk | contribs) |
|||
| (6 intermediate revisions by one other user not shown) | |||
| Line 1: | Line 1: | ||
| − | {{Definition|title= | + | {{Definition|title=polychlorinated naphthalenes (PCN) |
| − | |definition=Polychlorinated naphthalene (PCN) products are made by chemically reacting chlorine with [[naphthalene]]. A PCN can contain 1 to 8 chlorine atoms on 8 different positions, yielding 75 possible congeners. Commercial products are generally mixtures of several congeners and range from thin liquids | + | |definition=Polychlorinated naphthalene (PCN) products are made by chemically reacting chlorine with [[naphthalene]]. A PCN can contain 1 to 8 chlorine atoms on 8 different positions, yielding 75 possible congeners. Commercial products are generally mixtures of several congeners and range from thin liquids to high melting point solids<ref name="wik">http://en.wikipedia.org/wiki/Polychlorinated_naphthalene</ref>. }} |
== Notes == | == Notes == | ||
| Line 13: | Line 13: | ||
|- | |- | ||
| align="center" | C<sub>10</sub>H<sub>10-n</sub>Cl<sub>n</sub> | | align="center" | C<sub>10</sub>H<sub>10-n</sub>Cl<sub>n</sub> | ||
| − | + | with n=4: C<sub>10</sub>H<sub>6</sub>Cl<sub>4</sub> | |
|- | |- | ||
|} | |} | ||
Production of PCNs started in 1910, it peaked between 1930 and 1950. US production decreased from 3200 tonnes in 1957 to about 320 tonnes in 1978 due to the replacement of PCNs by substitutes. By 1980 US production had ceased. | Production of PCNs started in 1910, it peaked between 1930 and 1950. US production decreased from 3200 tonnes in 1957 to about 320 tonnes in 1978 due to the replacement of PCNs by substitutes. By 1980 US production had ceased. | ||
| − | PCNs have been mainly used in cable insulation, wood preservation, engine oil additives, electroplating masking compounds, capacitors and as a feedstock for dye production. The major sources of release of PCNs into the environment are likely from waste incineration (which also produces [[dioxins]]) and disposal of items containing chlorinated naphthalenes | + | PCNs have been mainly used in cable insulation, wood preservation, engine oil additives, electroplating masking compounds, capacitors and as a feedstock for dye production. The major sources of release of PCNs into the environment are likely to derive from waste incineration (which also produces [[dioxins]]) and disposal of items containing chlorinated naphthalenes<ref name="WHO">[http://www.inchem.org/documents/cicads/cicads/cicad34.htm#1.0 Howe, P.D.; C. Melber & J. Kielhorn et al. (2001), Chlorinated naphthalenes, International Programme on Chemical Safety CICAD 34]</ref>. |
| − | They have a low water solubility, and [[adsorption|adsorb]] to particles, soils and sediments. Highly chlorinated PCNs have a higher tendency to adsorb than those that are less chlorinated. They are stable chemicals although they can be biodegraded in the soil by micro-organisms. Chlorinated naphthalenes have been shown to be highly [[bioaccumulation|bioaccumulative]] in fish, but less so in shrimp and algae. The amount of bioaccumulation | + | They have a low water solubility, and [[adsorption|adsorb]] to particles, soils and sediments. Highly chlorinated PCNs have a higher tendency to adsorb than those that are less chlorinated. They are stable chemicals although they can be biodegraded in the soil by micro-organisms. Chlorinated naphthalenes have been shown to be highly [[bioaccumulation|bioaccumulative]] in [[pollution and benthic fishes|fish]], but less so in shrimp and algae. The amount of bioaccumulation increases with the degree of chlorination, although the highest chlorinated naphthalenes appear to bioaccumulate less. |
| − | PCNs are toxic to marine organisms. Some algae might show a reduced growth at concentrations above 0.1 mg/l. Concentrations of 80 µg/l | + | PCNs are [[toxic]] to marine organisms. Some algae might show a reduced growth at concentrations above 0.1 mg/l. Concentrations of 80 µg/l cause acute toxicity to horseshoe crabs. In work places PCN concentrations up to 14.5 mg/m<sup>3</sup> have been measured. These conditions caused severe skin rashes and liver conditions which led to the dead of workers<ref name="wik">http://en.wikipedia.org/wiki/Polychlorinated_naphthalene</ref>. |
| − | In fish, concentrations up to 300 µg/kg [[lipid weight]] of PCNs have been found. The concentrations in [[pollution and sea birds|sea birds]] are decreasing | + | In fish, concentrations up to 300 µg/kg [[lipid weight]] of PCNs have been found. The concentrations in [[pollution and sea birds|sea birds]] are decreasing<ref name="WHO">[http://www.inchem.org/documents/cicads/cicads/cicad34.htm#1.0 Howe, P.D.; C. Melber & J. Kielhorn et al. (2001), Chlorinated naphthalenes, International Programme on Chemical Safety CICAD 34]</ref>. |
<P> | <P> | ||
| Line 40: | Line 40: | ||
<references/> | <references/> | ||
| − | [[Category: | + | {{author |
| + | |AuthorID=19826 | ||
| + | |AuthorFullName=Daphnis De Pooter | ||
| + | |AuthorName=Daphnisd}} | ||
| + | |||
| + | [[Category:Toxicity chemicals]] | ||
Latest revision as of 13:34, 9 August 2020
Definition of polychlorinated naphthalenes (PCN):
Polychlorinated naphthalene (PCN) products are made by chemically reacting chlorine with naphthalene. A PCN can contain 1 to 8 chlorine atoms on 8 different positions, yielding 75 possible congeners. Commercial products are generally mixtures of several congeners and range from thin liquids to high melting point solids[1].
This is the common definition for polychlorinated naphthalenes (PCN), other definitions can be discussed in the article
|
Notes
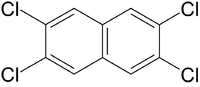
| 2,3,6,7-Tetrachlornaphthalin |
|---|
|
| Formula |
| C10H10-nCln
with n=4: C10H6Cl4 |
Production of PCNs started in 1910, it peaked between 1930 and 1950. US production decreased from 3200 tonnes in 1957 to about 320 tonnes in 1978 due to the replacement of PCNs by substitutes. By 1980 US production had ceased. PCNs have been mainly used in cable insulation, wood preservation, engine oil additives, electroplating masking compounds, capacitors and as a feedstock for dye production. The major sources of release of PCNs into the environment are likely to derive from waste incineration (which also produces dioxins) and disposal of items containing chlorinated naphthalenes[2].
They have a low water solubility, and adsorb to particles, soils and sediments. Highly chlorinated PCNs have a higher tendency to adsorb than those that are less chlorinated. They are stable chemicals although they can be biodegraded in the soil by micro-organisms. Chlorinated naphthalenes have been shown to be highly bioaccumulative in fish, but less so in shrimp and algae. The amount of bioaccumulation increases with the degree of chlorination, although the highest chlorinated naphthalenes appear to bioaccumulate less.
PCNs are toxic to marine organisms. Some algae might show a reduced growth at concentrations above 0.1 mg/l. Concentrations of 80 µg/l cause acute toxicity to horseshoe crabs. In work places PCN concentrations up to 14.5 mg/m3 have been measured. These conditions caused severe skin rashes and liver conditions which led to the dead of workers[1].
In fish, concentrations up to 300 µg/kg lipid weight of PCNs have been found. The concentrations in sea birds are decreasing[2].
Environmental standards and legislation
Included in the OSPAR list of substances of priority action
References
Please note that others may also have edited the contents of this article.
|