Difference between revisions of "Chlorpyrifos"
(→Notes) |
Dronkers J (talk | contribs) |
||
| (10 intermediate revisions by one other user not shown) | |||
| Line 1: | Line 1: | ||
| − | + | {{Tocright}} | |
| − | {{Definition|title= | + | {{Definition|title=chlorpyrifos |
| − | |definition=Chlorpyrifos is an insecticide that has been widely used to control cockroaches, fleas, termites and to control crop pests. In 1997, chlorpyrifos was withdrawn from most indoor and pet uses. Chlorpyrifos is a white crystal-like solid with a strong odour | + | |definition=Chlorpyrifos is an insecticide that has been widely used to control cockroaches, fleas, termites and to control crop pests. In 1997, chlorpyrifos was withdrawn from most indoor and pet uses. Chlorpyrifos is a white crystal-like solid with a strong odour<ref name="US">[http://www.atsdr.cdc.gov/toxprofiles/tp84.pdf U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES 1997 TOXICOLOGICAL PROFILE FOR CHLORPYRIFOS]</ref>. }} |
== Notes == | == Notes == | ||
| Line 17: | Line 17: | ||
|} | |} | ||
| − | Chlorpyrifos enters the environment through direct application to crops, lawns and domesticated animals. | + | Chlorpyrifos enters the environment through direct application to crops, lawns and domesticated animals. It may also enter the environment through spills and the disposal of chlorpyrifos waste. |
| − | It has a low water solubility (0.7 mg/l) and a high tendency to [[adsorption|adsorb]] to particles and soils. Therefore it's unlikely to end up in the marine ecosystem. It has a high volatility causing it after use to usually evaporate into the atmosphere , where it is degraded rapidly. It, however, only evaporates slowly from water bodies, mainly because | + | It has a low water solubility (0.7 mg/l) and a high tendency to [[adsorption|adsorb]] to particles and soils. Therefore it's unlikely to end up in the marine ecosystem. It has a high [[volatile|volatility]] causing it after use to usually evaporate into the atmosphere , where it is degraded rapidly. It, however, only evaporates slowly from water bodies, mainly because, in water, chlorpyrifos is associated to suspended particles. In water it is slowly hydrolysed with a [[half-life]] of less than 60 days. <ref name="US">[http://www.atsdr.cdc.gov/toxprofiles/tp84.pdf U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES 1997 TOXICOLOGICAL PROFILE FOR CHLORPYRIFOS]</ref> |
| − | It has a high potential towards [[bioaccumulation]] and might possibly also [[biomagnification|biomagnify]] through [[food chain|food chains]] | + | It has a high potential towards [[bioaccumulation]] and might possibly also [[biomagnification|biomagnify]] through [[food chain|food chains]]<ref name="US">[http://www.atsdr.cdc.gov/toxprofiles/tp84.pdf U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES 1997 TOXICOLOGICAL PROFILE FOR CHLORPYRIFOS]</ref>. |
| − | Chlorpyrifos is very toxic for crustaceans, especially amphipods which die | + | Chlorpyrifos is very [[toxic]] for crustaceans, especially [http://www.marinespecies.org/aphia.php?p=taxdetails&id=1135 amphipods] which die when exposed to concentrations above 0.1 µg/l. Concentrations which cause acute toxicity in [[pollution and pelagic fishes|fish]] range, depending on the [[species]], from 1 µg/l to 500 µg/l<ref>[http://www.pesticideinfo.org/List_AquireAcuteSum.jsp?Rec_Id=PC33392 www.pesticideinfo.org August 20 2009]</ref>. |
| − | In humans, chlorpyrifos can cause cholinesterase inhibition; it can overstimulate the nervous system causing nausea, dizziness, confusion, and at very high exposures respiratory paralysis and death | + | In humans, chlorpyrifos can cause cholinesterase inhibition; it can overstimulate the nervous system causing nausea, dizziness, confusion, and at very high exposures, respiratory paralysis and death<ref>[http://www.epa.gov/oppsrrd1/REDs/chlorpyrifos_ired.pdf U.S. EPA 2002 Interim Reregistration Eligibility Decision for Chlorpyrifos]</ref>. |
<P> | <P> | ||
<BR> | <BR> | ||
| Line 44: | Line 44: | ||
==References== | ==References== | ||
<references/> | <references/> | ||
| + | {{author | ||
| + | |AuthorID=19826 | ||
| + | |AuthorFullName=Daphnis De Pooter | ||
| + | |AuthorName=Daphnisd}} | ||
| − | [[Category: | + | [[Category:Toxicity chemicals]] |
Latest revision as of 13:05, 9 August 2020
Definition of chlorpyrifos:
Chlorpyrifos is an insecticide that has been widely used to control cockroaches, fleas, termites and to control crop pests. In 1997, chlorpyrifos was withdrawn from most indoor and pet uses. Chlorpyrifos is a white crystal-like solid with a strong odour[1].
This is the common definition for chlorpyrifos, other definitions can be discussed in the article
|
Notes
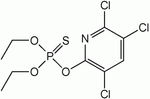
| Chlorpyrifos |
|---|
|
| Formula |
| C9H11Cl13NO3PS |
Chlorpyrifos enters the environment through direct application to crops, lawns and domesticated animals. It may also enter the environment through spills and the disposal of chlorpyrifos waste.
It has a low water solubility (0.7 mg/l) and a high tendency to adsorb to particles and soils. Therefore it's unlikely to end up in the marine ecosystem. It has a high volatility causing it after use to usually evaporate into the atmosphere , where it is degraded rapidly. It, however, only evaporates slowly from water bodies, mainly because, in water, chlorpyrifos is associated to suspended particles. In water it is slowly hydrolysed with a half-life of less than 60 days. [1]
It has a high potential towards bioaccumulation and might possibly also biomagnify through food chains[1].
Chlorpyrifos is very toxic for crustaceans, especially amphipods which die when exposed to concentrations above 0.1 µg/l. Concentrations which cause acute toxicity in fish range, depending on the species, from 1 µg/l to 500 µg/l[2]. In humans, chlorpyrifos can cause cholinesterase inhibition; it can overstimulate the nervous system causing nausea, dizziness, confusion, and at very high exposures, respiratory paralysis and death[3].
Environmental standards and legislation
Included in the water framework list of priority substances
See also
Chlorpyrifos on the ED North Database
References
Please note that others may also have edited the contents of this article.
|