Difference between revisions of "Anthracene"
(Next paragraph) |
Dronkers J (talk | contribs) |
||
| (19 intermediate revisions by 4 users not shown) | |||
| Line 1: | Line 1: | ||
| + | {{Tocright}} | ||
| + | {{ Definition|title= anthracene | ||
| + | |||
| + | |definition= Anthracene forms part of a wide group of [[PAH|polyaromatic hydrocarbons]]. Of this group antracene is one of the more toxic ones<ref>Kennish, M. J. (1996): Practical Handbook of Estuarine and Marine Pollution, CRC Press 524 pp</ref>. }} | ||
| + | |||
| + | |||
| + | == Notes == | ||
| + | |||
| + | ===Natural state and human emission=== | ||
| + | |||
{| class="toccolours" border="1" style="float: right; clear: right; margin: 0 0 1em 1em; border-collapse: collapse;" | {| class="toccolours" border="1" style="float: right; clear: right; margin: 0 0 1em 1em; border-collapse: collapse;" | ||
! bgcolor="#FF8888" | Anthracene | ! bgcolor="#FF8888" | Anthracene | ||
| Line 9: | Line 19: | ||
|- | |- | ||
|} | |} | ||
| − | + | Anthracene is used as an intermediate compound for the manufacturing of dyes and polyradicals used to make resins. It may also be used as a diluent for wood protection products, an insecticide, or a fungicide. Furthermore, anthracene is an organic cristallized photoconductor used in electrophotography. | |
| − | |||
| − | |||
| − | |||
In the nature, anthracene can be found to a small degree in fossil fuels (12 g/kg of coal). It is also present in fuel (100 to 300 mg/L) and petrol (1.55 mg/L, to 2,6 mg/L in high-octane petrols)<ref>Verschueren K. (1996) - Anthracene. Handbook of Environmental Data on Organic Chemicals. | In the nature, anthracene can be found to a small degree in fossil fuels (12 g/kg of coal). It is also present in fuel (100 to 300 mg/L) and petrol (1.55 mg/L, to 2,6 mg/L in high-octane petrols)<ref>Verschueren K. (1996) - Anthracene. Handbook of Environmental Data on Organic Chemicals. | ||
| Line 18: | Line 25: | ||
The main sources of anthropic emissions are the following: | The main sources of anthropic emissions are the following: | ||
| − | * tailpipes of car motors (0.02 to 6.45 μg/m<sup>3</sup>)<ref>OMS IPCS (1998) - Environmental Health Criteria 202: Selected Non-Heterocyclic Policyclic Aromatic Hydrocarbons. World Health Organisation, International Programme on Chemical Safety. | + | * tailpipes of car motors (0.02 to 6.45 μg/m<sup>3</sup>)<ref>OMS IPCS (1998) - Environmental Health Criteria 202: Selected Non-Heterocyclic Policyclic Aromatic Hydrocarbons. World Health Organisation, International Programme on Chemical Safety.</ref>; |
* cokefaction and gaseifaction of coal and more generally emissions of coal kilns and fuel kilns; | * cokefaction and gaseifaction of coal and more generally emissions of coal kilns and fuel kilns; | ||
* purification of petrol; | * purification of petrol; | ||
| Line 26: | Line 33: | ||
* combustion of pneumatic wastes (rubber). | * combustion of pneumatic wastes (rubber). | ||
| − | ==Behaviour== | + | ===Behaviour=== |
| − | In water, anthracene is easily adsorbed on particles in suspension. However, it may also volatilize | + | In water, anthracene is easily [[adsorption|adsorbed]] on particles in suspension. However, it may also [[volatile|volatilize]], which means its final amount is the result of two processes in competition. In soils, anthracene does not move very much: it may volatilize from moist soils, but only very little from dry ones.<ref name="hsdb">[http://www.toxnet.nlm.nih.gov HSDB (2001) - Polycyclic Aromatic Hydrocarbons - Anthracene. Hazardous Substances Data Bank, National Library of Medicine. ]</ref> |
| − | Bank, National Library of Medicine. | ||
In distilled water, anthracene is destroyed by photolysis in several hours when it is exposed to natural light. However, the substance is not biodegradable: after 28 days in living organisms, only 1.9% of the substance is destroyed.<ref>Callahan M.A., Slimak M.W., Gabel N.W., May I.P., Fowler C.F., Freed J.R., Jennings P., Durfee R.L., Whitmore F.C., Maestri W.R., Mabey B.R. and Holt B.R. (1979) - Water-related environmental fate of 129 priority pollutants Volume II. Halogenated aliphatic hydrocarbons, halogenated ethers, monocyclic aromatics, phtalates esters, polycyclic aromatic hydrocarbons, nitrosamines, miscellaneous compounds. United States Environmental Protection Agency. Washington, DC. EPA-440/4-79-029b.</ref> | In distilled water, anthracene is destroyed by photolysis in several hours when it is exposed to natural light. However, the substance is not biodegradable: after 28 days in living organisms, only 1.9% of the substance is destroyed.<ref>Callahan M.A., Slimak M.W., Gabel N.W., May I.P., Fowler C.F., Freed J.R., Jennings P., Durfee R.L., Whitmore F.C., Maestri W.R., Mabey B.R. and Holt B.R. (1979) - Water-related environmental fate of 129 priority pollutants Volume II. Halogenated aliphatic hydrocarbons, halogenated ethers, monocyclic aromatics, phtalates esters, polycyclic aromatic hydrocarbons, nitrosamines, miscellaneous compounds. United States Environmental Protection Agency. Washington, DC. EPA-440/4-79-029b.</ref> | ||
| − | Anthracene may also | + | Anthracene may also [[bioaccumulation|bioaccumulate]] in aquatic organisms, as in some organisms it can be absorbed 10.000 times faster than eliminated. <ref>Spacie A., Landrum P. and Leversee G. (1983) - Uptake, depuration, and biotransformation of anthracene and benzo[a]pyrene in bluegill sunfish. Ecotoxicol Environ Saf, 7, 330-341.</ref><ref>Linder G., Bergman H. and Meyer J. (1985) - Anthracene bioconcentration in rainbow-trout during single-compound and complex-mixture exposures. Environ Toxicol Chem, 4, 549-558.</ref><ref>CITI (1992) - Biodegradation and Bioaccumulation data of existing chemicals based on the CSCL Japan. Chemicals Inspection and Testing Institute. Japan. October 1992.</ref> |
| + | |||
| + | ===Effects on health=== | ||
| + | |||
| + | One of the most common ways to be contaminated by anthracene is through breathing contaminated air. One can also ingest anthracene by eating food containing anthracene or drinking [[pollution|polluted]] water. A third source of exposure is direct contact of the skin with contaminated soil or products like heavy oils, coal tar, roofing tar or creosote (oily liquid found in coal tar and used to preserve wood). Target organs affected by anthracene include the kidneys, the liver and fat. | ||
| + | |||
| + | Once inside a body, anthracene seems to target skin, blood, stomach, intestines and the lymph system. Exposure to high doses of anthracene at a short time can cause damage to the skin, like burning, itching and edema and a build up of fluid in tissues. Humans exposed to anthracene experienced headaches, nausea, loss of appetite, inflammation or swelling of the stomach and intestines. In addition, their reaction time slowed and they felt weak. | ||
| − | + | Other effects have been found to occur on animals, like reproductive problems, birth defects, and damage to the immune system. These effects have not yet been demonstrated on humans. | |
| + | It also remains to be proven whether anthracene is a cancer causing substance. | ||
| + | <P> | ||
| + | <BR> | ||
| + | <P> | ||
| + | |||
| + | == Environmental standards and legislation == | ||
| + | |||
| + | [[OSPAR List of priority substances|Included (as polyaromatic hydrocarbons) in the OSPAR list of substances of priority action]] | ||
| + | |||
| + | [[List of priority substances|Included in the water framework list of priority substances]] | ||
| + | <P> | ||
| + | <BR> | ||
| + | <P> | ||
| + | |||
| + | == See also == | ||
| + | |||
| + | [http://www.vliz.be/projects/endis/EDnorth.php?showchemprop=true&showeffects=true&chemeffects=true&chemid=346 Anthracene on the ED North Database] | ||
| + | |||
| + | [http://www.ospar.org/documents%5Cdbase%5Cpublications%5Cp00137_BD%20on%20PAHs.pdf OSPAR background document on polyaromatic hydrocarbons] | ||
| + | <P> | ||
| + | <BR> | ||
| + | <P> | ||
==References== | ==References== | ||
<references/> | <references/> | ||
| + | |||
| + | |||
| + | [[Category:Toxicity chemicals]] | ||
Latest revision as of 13:01, 9 August 2020
Definition of anthracene:
Anthracene forms part of a wide group of polyaromatic hydrocarbons. Of this group antracene is one of the more toxic ones[1].
This is the common definition for anthracene, other definitions can be discussed in the article
|
Notes
Natural state and human emission
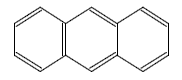
| Anthracene |
|---|
|
| Formula |
| C14H10 |
Anthracene is used as an intermediate compound for the manufacturing of dyes and polyradicals used to make resins. It may also be used as a diluent for wood protection products, an insecticide, or a fungicide. Furthermore, anthracene is an organic cristallized photoconductor used in electrophotography.
In the nature, anthracene can be found to a small degree in fossil fuels (12 g/kg of coal). It is also present in fuel (100 to 300 mg/L) and petrol (1.55 mg/L, to 2,6 mg/L in high-octane petrols)[2].
The main sources of anthropic emissions are the following:
- tailpipes of car motors (0.02 to 6.45 μg/m3)[3];
- cokefaction and gaseifaction of coal and more generally emissions of coal kilns and fuel kilns;
- purification of petrol;
- use of impregnation oils for wood treatment;
- production of asphalt for roads covering;
- smoke of wood coal;
- combustion of pneumatic wastes (rubber).
Behaviour
In water, anthracene is easily adsorbed on particles in suspension. However, it may also volatilize, which means its final amount is the result of two processes in competition. In soils, anthracene does not move very much: it may volatilize from moist soils, but only very little from dry ones.[4]
In distilled water, anthracene is destroyed by photolysis in several hours when it is exposed to natural light. However, the substance is not biodegradable: after 28 days in living organisms, only 1.9% of the substance is destroyed.[5]
Anthracene may also bioaccumulate in aquatic organisms, as in some organisms it can be absorbed 10.000 times faster than eliminated. [6][7][8]
Effects on health
One of the most common ways to be contaminated by anthracene is through breathing contaminated air. One can also ingest anthracene by eating food containing anthracene or drinking polluted water. A third source of exposure is direct contact of the skin with contaminated soil or products like heavy oils, coal tar, roofing tar or creosote (oily liquid found in coal tar and used to preserve wood). Target organs affected by anthracene include the kidneys, the liver and fat.
Once inside a body, anthracene seems to target skin, blood, stomach, intestines and the lymph system. Exposure to high doses of anthracene at a short time can cause damage to the skin, like burning, itching and edema and a build up of fluid in tissues. Humans exposed to anthracene experienced headaches, nausea, loss of appetite, inflammation or swelling of the stomach and intestines. In addition, their reaction time slowed and they felt weak.
Other effects have been found to occur on animals, like reproductive problems, birth defects, and damage to the immune system. These effects have not yet been demonstrated on humans.
It also remains to be proven whether anthracene is a cancer causing substance.
Environmental standards and legislation
Included (as polyaromatic hydrocarbons) in the OSPAR list of substances of priority action
Included in the water framework list of priority substances
See also
Anthracene on the ED North Database
OSPAR background document on polyaromatic hydrocarbons
References
- ↑ Kennish, M. J. (1996): Practical Handbook of Estuarine and Marine Pollution, CRC Press 524 pp
- ↑ Verschueren K. (1996) - Anthracene. Handbook of Environmental Data on Organic Chemicals. New York, Van Nostrand Reinhold Co. 3rd Ed, pp. 214-217
- ↑ OMS IPCS (1998) - Environmental Health Criteria 202: Selected Non-Heterocyclic Policyclic Aromatic Hydrocarbons. World Health Organisation, International Programme on Chemical Safety.
- ↑ HSDB (2001) - Polycyclic Aromatic Hydrocarbons - Anthracene. Hazardous Substances Data Bank, National Library of Medicine.
- ↑ Callahan M.A., Slimak M.W., Gabel N.W., May I.P., Fowler C.F., Freed J.R., Jennings P., Durfee R.L., Whitmore F.C., Maestri W.R., Mabey B.R. and Holt B.R. (1979) - Water-related environmental fate of 129 priority pollutants Volume II. Halogenated aliphatic hydrocarbons, halogenated ethers, monocyclic aromatics, phtalates esters, polycyclic aromatic hydrocarbons, nitrosamines, miscellaneous compounds. United States Environmental Protection Agency. Washington, DC. EPA-440/4-79-029b.
- ↑ Spacie A., Landrum P. and Leversee G. (1983) - Uptake, depuration, and biotransformation of anthracene and benzo[a]pyrene in bluegill sunfish. Ecotoxicol Environ Saf, 7, 330-341.
- ↑ Linder G., Bergman H. and Meyer J. (1985) - Anthracene bioconcentration in rainbow-trout during single-compound and complex-mixture exposures. Environ Toxicol Chem, 4, 549-558.
- ↑ CITI (1992) - Biodegradation and Bioaccumulation data of existing chemicals based on the CSCL Japan. Chemicals Inspection and Testing Institute. Japan. October 1992.