Difference between revisions of "Methylmercury"
(→Notes) |
(→See also) |
||
| (53 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
| − | {{ | + | {{Definition|title= methylmercury |
| − | Definition|title= | + | |definition= Soluble and highly toxic compound of [[mercury]] formed in the environment by microbial methylation of [[mercury]]. <ref>Lawrence E (ed.), 2000. Henderson’s Dictionary of Biological Terms. 12th edition. Prentice Hall, Pearson Education Limited. Harlow, Great Britain.</ref> }} |
| − | |definition= Soluble and highly toxic compound of mercury formed in the environment by microbial methylation of mercury. <ref>Lawrence E (ed.), 2000. Henderson’s Dictionary of Biological Terms. 12th edition. Prentice Hall, Pearson Education Limited. Harlow, Great Britain.</ref> }} | ||
== Notes == | == Notes == | ||
| − | |||
| − | == | + | {| class="toccolours" border="1" style="float: right; clear: right; margin: 0 0 1em 1em; border-collapse: collapse;" |
| + | ! bgcolor="#FF8888" | methylmercury | ||
| + | |- | ||
| + | | align="center" bgcolor="#FFFFFF" | [[Image:methylmercury.png|200px|methylmercury]] | ||
| + | |- | ||
| + | | align="center" | X<sup>-</sup> represents a random ion | ||
| + | |- | ||
| + | |} | ||
| + | While inorganic mercury is the dominant form, most mercury which accumulates in [[benthic]] invertabrates and [[Pollution and benthic fishes| fish]] is methylmercury. Unlike most other metals, methylmercury [[biomagnification|biomagnifies]] through the food chain. As such methylmercury is expected to be most hazardous for organisms on the top of the food chain. Biomagnification causes high trophic level [[pollution and benthic fishes|carnivorous fish]] (like mackerel and tuna) to contain 10.000 times more methylmercury than the surrounding environment. Species eating these fish humans, [[Pollution and sea birds|sea birds]] and [[Pollution and marine mammals|marine mammals]]) are expected to experience even more problems. However while this holds true for humans, marine birds and marine mammals appear to be quite resistant towards methylmercury accumulation. | ||
| + | |||
| + | Humans acquire methylmercury through consumption of fish and mussels. Usually this doesn't pose any health risks, although high consumption of predatory fish (with high mercury content) by pregnant women, might cause problems for their young and unborn babies. | ||
| + | Methylmercury poisoning by consumption of sea food has caused [[minamata disease|human deaths in Japan]]. The consumed fish had mercury concentrations of 10-55 ppm (parts per million [[dry weight]]), most of it in the form of methylmercury. | ||
| + | |||
| + | Today public health standards for mercury in fish are typically between 0,5 and 1ppm. The limit of 1 ppm generally applies to species for which a standard of 1 ppm would exclude a large proportion of the catch from the market. The limit of 1 ppm is typically coupled with the advise to to the public to [http://www.nrdc.org/health/effects/mercury/walletcard.pdf limit its consumption of these species.]<ref>Clark, R,B., 1999. Marine pollution. Oxford University press, Fourth edition, pp 161</ref> | ||
| + | You can also verify whether the fish you eat has been caught in a [http://www.goedevis.nl/Englishpage/view sustainable manner].<ref>http://www.lenntech.com/Periodic-chart-elements/Hg-en.htm</ref> | ||
| + | |||
| + | |||
| + | |||
| + | == See also == | ||
| + | *[[Mercury]] | ||
| + | *[[Mercury pollution]] | ||
| + | *[[Minamata disease]] | ||
| + | <P> | ||
| + | <BR> | ||
| + | <P> | ||
| + | |||
| + | ==References== | ||
<references/> | <references/> | ||
| + | {{author | ||
| + | |AuthorID=19826 | ||
| + | |AuthorFullName=Daphnis De Pooter | ||
| + | |AuthorName=Daphnisd}} | ||
Latest revision as of 15:49, 20 March 2013
Notes
| methylmercury |
|---|
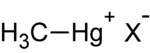
|
| X- represents a random ion |
While inorganic mercury is the dominant form, most mercury which accumulates in benthic invertabrates and fish is methylmercury. Unlike most other metals, methylmercury biomagnifies through the food chain. As such methylmercury is expected to be most hazardous for organisms on the top of the food chain. Biomagnification causes high trophic level carnivorous fish (like mackerel and tuna) to contain 10.000 times more methylmercury than the surrounding environment. Species eating these fish humans, sea birds and marine mammals) are expected to experience even more problems. However while this holds true for humans, marine birds and marine mammals appear to be quite resistant towards methylmercury accumulation.
Humans acquire methylmercury through consumption of fish and mussels. Usually this doesn't pose any health risks, although high consumption of predatory fish (with high mercury content) by pregnant women, might cause problems for their young and unborn babies. Methylmercury poisoning by consumption of sea food has caused human deaths in Japan. The consumed fish had mercury concentrations of 10-55 ppm (parts per million dry weight), most of it in the form of methylmercury.
Today public health standards for mercury in fish are typically between 0,5 and 1ppm. The limit of 1 ppm generally applies to species for which a standard of 1 ppm would exclude a large proportion of the catch from the market. The limit of 1 ppm is typically coupled with the advise to to the public to limit its consumption of these species.[2] You can also verify whether the fish you eat has been caught in a sustainable manner.[3]
See also
References
- ↑ Lawrence E (ed.), 2000. Henderson’s Dictionary of Biological Terms. 12th edition. Prentice Hall, Pearson Education Limited. Harlow, Great Britain.
- ↑ Clark, R,B., 1999. Marine pollution. Oxford University press, Fourth edition, pp 161
- ↑ http://www.lenntech.com/Periodic-chart-elements/Hg-en.htm
Please note that others may also have edited the contents of this article.
|