Difference between revisions of "Atrazine"
() |
|||
| Line 2: | Line 2: | ||
{{Definition|title= atrazine | {{Definition|title= atrazine | ||
| − | |definition= Atrazine is an organic compound which is widely used as a herbicide. Its use is controversial due to its effects on nontarget species, such as on amphibians. Its use was banned in the European Union in 2004 but it remains one of the most widely used herbicides in the U.S. . <ref name="Wiki">[http://en.wikipedia.org/wiki/Atrazine www.wikipedia.org 13 | + | |definition= Atrazine is an organic compound which is widely used as a herbicide. Its use is controversial due to its effects on nontarget species, such as on amphibians. Its use was banned in the European Union in 2004 but it remains one of the most widely used herbicides in the U.S. . <ref name="Wiki">[http://en.wikipedia.org/wiki/Atrazine www.wikipedia.org August 13 2009]</ref> }} |
== Notes == | == Notes == | ||
Revision as of 09:42, 27 August 2009
Definition of atrazine:
Atrazine is an organic compound which is widely used as a herbicide. Its use is controversial due to its effects on nontarget species, such as on amphibians. Its use was banned in the European Union in 2004 but it remains one of the most widely used herbicides in the U.S. . [1]
This is the common definition for atrazine, other definitions can be discussed in the article
|
Notes
| Atrazine |
|---|
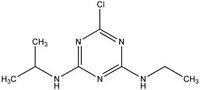
|
| Formula |
| C8H14ClN5 |
Atrazine was first used in the United States in 1959. It currently still is one of the most widely used agricultural pesticides in the United States, with an annual production of over 35.000 tonnes. It is mainly used in sorghum, corn and sugarcane cultivation. It is released in the environment both from production sites as from its use as a herbicide.
Atrazine has a moderate water solubility of 30 mg/l and is therefore mobile the environment. It is also persistent; in water it takes over 150 days to half its concentration, it takes even longer in the soil. It has a relative low tendency to adsorb to soils or particles. As such, atrazine is expected to be present in surface water and groundwater. It also has a low tendency to evaporate to the atmosphere and can afterwards be deposited by rainfall, although it is rapidly degraded in the atmosphere.
It has a very low tendency to bioaccumulate and is therefore not expected to biomagnify through food chains.
It has a low toxicity in crustaceans and fish; concentrations above 3mg/l are necessary to induce lethal effects. Some fish and crustacean species can even survive in waters with more than 80 mg/l of Atrazine. Phytoplancton is more vulnerable and can be affected by concentrations of only 60 µg/l. There have been reports that low concentrations of only 20 µg/l could change the behaviour of amphibians. Atrazine has also been suspected to induce endocrine disrupting effects in amphibians and mammals. [2]
Environmental standards and legislation
Included in the water framework list of priority substances
See also