Coral reefs
This article describes the habitat and functions of coral reefs. It is one of the sub-categories within the section dealing with biodiversity of marine habitats and ecosystems.
Contents
[hide]Introduction

Coral reefs are among the most diverse ecosystems in the world. Corals belong to the Phylum Cnidaria. The best-known type of corals is the one living in clear, warm tropical waters with plenty of colorful fishes (Fig.1). This is a rocky, shallow water type. The water is clear because concentrations of nutrients are typically low. Besides, there are also deep water corals that live in dark cold waters and soft corals that live in shallow, cold waters. Corals are wave resistant rock structures, created by calcium carbonate secreting animals and plants. Coral reefs provide the physical foundation of a number of mid-ocean atoll nations (e.g., Kiribati, Tuvalu, Maldives, and Marshall Islands). Coral reefs also protect coral islands and island populations against the destructive forces of the sea under storm conditions, see Coral islands.
Formation (warm water corals)
The formation of corals begins when free-swimming coral larvae attach to submerged rocks (e.g. sinking volcanic islands) or other hard substrate along the edges of islands or continents. The reef expands and will form an atoll, barrier or fringing reef. These 3 are the main reef types. Atolls are circular or oval coral reefs (Fig. 2a) that partially or completely encircle a lagoon. A barrier reef (Fig. 2b) is a reef that borders the shoreline over a long distance. They are separated from the adjacent land mass by a lagoon. A fringing reef (Fig. 2c) forms borders along the shoreline and the surrounding islands, but at shorter distance than barrier reefs. It is directly attached to the land. This is the most common type of coral reefs [2]. Other special reef arrangements are apron, patch, ribbon, table and bank reefs. Apron reefs are very similar to fringing reefs. Both are extending downward from the land margin, the former more gently sloping than the latter. Patch reefs (Fig. 2d) are small, isolated outcrops of coral surrounded by sand and/or seagrass, and may form part of a fringing reef system. Ribbon reefs (Fig. 2e) are the small and long components of a barrier or are connected to an atoll lagoon. Table reefs are future atolls, but are not yet connected to a lagoon. Finally, bank reefs resemble the patch reefs, but are larger and often semi-circular.
Coral reefs are among the oldest habitats in the ocean. They have slow growth rates and it may take up to 10,000 years for a coral reef to fully develop from the first colonizing larvae [3]. The different types of coral reefs all share similarities in their biogeographic profiles. Horizontal and vertical zonation depends on bottom topography, depth, wave and current strength, light, temperature and suspended sediments.
Distribution
Warm water corals (WWC)
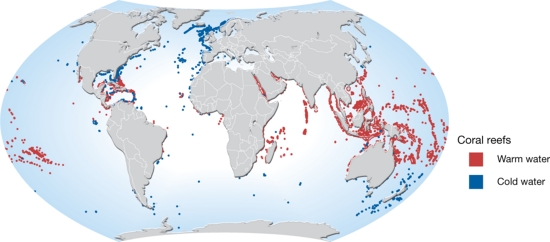
The warm water corals are found in the tropics (between 30°N and 30°S, Fig. 3) in areas where the water is clear and over 18°C. Consequently, corals are typically absent along the western margins of Africa and South America where cool water upwelling prevails. The maximum depth for warm water corals is generally around 60 meters. Some species of coral are found in all the world's oceans. Tropical coral ecosystems cover about 300,000 km2 of the Earth’s surface.
Cold-water corals (CWC)
Globally, cold-water corals (CWC) cover a wide range of depths (40 - 2000 m), water temperatures of 4-10 °C and latitudes (70°N – 60°S, Fig. 3). They are most common on continental slopes, on deep shelves and along the flanks of oceanic banks and seamounts, and are found around vents, in fjords and around offshore submarine banks (Cordes et al., 2016[4]). Submarine canyons are hotspots of cold-water corals by providing exposed hard and steep substrate and strong bottom currents that transport organic matter from the continental shelf to the deep sea[5]. The majority of CWCs occur between depths of 200 to 1000 m, with the bathymetric ranges becoming shallower towards the poles (Roberts et al., 2009[6]).
Lophelia pertusa (Fig. 8) is the dominant reef-forming CWC in the Atlantic and is found at 330-470 m water depth (Hebbeln et al., 2020[7]). The suitable temperature ranges from 4°C to 13°C, but they occur in some regions at much higher temperatures (>20°C). Lophelia is not found in the polar regions. Lophelia thrives in hypoxic water (lower oxygen limit DO ~4 ml/l but in some areas ~1 ml/l) (Hebbeln et al, 2020[7]) that is saturated with aragonite. The other important reef forming cold water coral, the fragile zigzag coral Madrepora oculata, is found in the north-east and western Atlantic and the Mediterranean Sea. Gorgonians (soft corals) are a major cold water species in the South China Sea[8].
Biology
The principal reef-building organisms are hard coral (scleractinian corals). The formation starts with a larva known as planula that settles down and attaches itself to a hard substrate. Then the larva develops into a coral polyp and secretes calcium carbonate around its body. The reproduction is by budding, an asexual process. As the asexual reproduction continues, the colony grows. Some polyps in the colony develop gonads and are able to reproduce sexually. These polyps release sperm and eggs in the surrounding water. To help with fertilization, both eggs and sperm cells have protein molecules on their surfaces to identify other cells of the same species (Karleskint, 1998[10]).
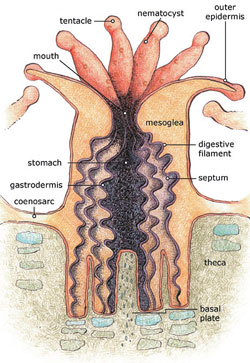
The coral polyp has a sac-like structure that is protected by a rigid calcium-carbonate exoskeleton (Fig. 4). This is called a corallite. The bottom of the corallite is divided into vertical segments or septa. At the top, the polyp has an opening that is a combined mouth and anus. This leads to the gut. The opening is surrounded by tentacles with mucus secreting cells for catching prey. The single-celled dinoflagellates Zooxanthellae are embedded in the outer layer of the coral’s flesh (epidermis), giving the coral it's color (Fig. 5). This provides a stable environment for them. The cells are abundant and can represent up to 75% of the tissue weight. The zooxanthellae require sunlight for photosynthesis. They provide nutrients to the polyps and reduce the level of carbon dioxide. This makes the conditions for the formation of skeletons more suitable. In turn, the zooxanthellae get a suitable habitat. The coral species which harbour the zooxanthellae are called hermatypic.
Not all corals are reef building species. There are also hard corals existing as single, solitary polyps. Some temperate species form small colonies only. Corals that lack the hard outer covering of calcium carbonate are soft corals. Cold water corals lack the symbiotic dinoflagellates. The species without zooxanthellae is called ahermatypic.
Reef zonation (WWC)
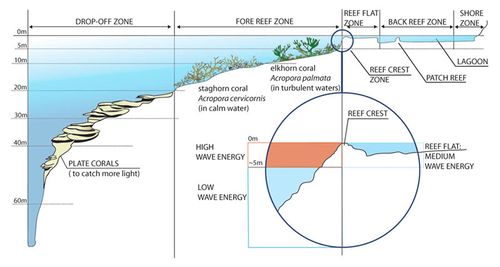
On the seaward side, the reef rises from the lower depths of the ocean to a level just at or just below the surface of the water (Fig. 6). This is called the reef front or fore-reef. The slope of this zone varies from gentle to steep. It sometimes forms a vertical wall known as a drop-off. But generally, the reef front forms finger-like arrangements called spur and groove formations. It breaks the wave energy and prevents damage to the reef. The grooves are sand-filled pockets, which allow sediment to be channelled down and away from the coral surface and provide a habitat for many species of burrowing organisms. The reef crest is the highest point of the reef. More land inward, the reef flat or back reef is formed. This zone has a high variability. The bottom of the reef flat consists of rock, sand, coral cobble or a combination of these. Seagrasses are commonly found in this zone. The reef ends at the shoreline or descends into the lagoon.
The different levels in the zonation support different species. On the on the intermediate slopes of the reef front, dome-shaped, massive brain corals (Diploria, Fig. 7a) and columnar pillar corals (Dendrogyra, Fig. 7b) are found. Below this region, plate corals such as Pectinia, Pavona and Agaricia are found. Higher upon the reef, where wave stress is greatest, there are branched species of coral. A coral occuring in this zone is the elkhorn coral (Acropora palmata, Fig. 7c). Behind the reef front, more protected zones are occupied with more delicate species such as the staghorn coral (Acropora cervicornis, Fig. 7d), finger (Stylophora), cluster (Pocillopora) and lace corals (Pocillopora damicornis). In shallow, calmer waters away from the reef front, small species such as rose (Meandrina, Manicina), flower (Mussa, Eusmilia) and star (Montastraea) corals are found. [10]
Fig. 7a. Brain coral (Diploria strigosa) [11]
Fig. 7b. Pillar coral (Dendrogyra cylindrus) [12]
Fig. 7c. Elkhorn coral (Acropora palmata)
Fig. 7d. Staghorn coral (Acropora cervicornis) Photo credit W. Jaap
Conditions for growth
Warm water corals
Reef-building, warm water corals need specific environmental conditions to grow:
- Clear water, allowing light to reach the photosynthetic zooxanthellae. Light-absorbing adaptations enable some species to live in dim blue light
- Ocean temperatures above 18°C; the optimum temperature for most corals is 26–27 °C. Many coral species only survive within a narrow temperature window.
- Energetic wave action which ensures supply of food and oxygen, the distribution of larvae and which prevents sediment to settle on the reefs
- Precipitation of calcium (aragonite) necessary for the formation of skeletons
- Low carbon dioxide concentrations; acid dissolution as a result of ocean acidification can cause loss of reef structure
- Hard substrate
Coral reefs are generally absent from river mouths that have characteristically low salinity levels and high sediment supply. Corals thrive in low-nutrient waters; high-nutrient levels can severely inhibit coral production[13].
Cold water corals

Cold water corals (CWCs) are organisms belonging to the cnidarian orders Scleractinia (stony corals), Alcyonacea (soft corals, including gorgonians), Antipatharia (black corals), Pennatulacea (seapens) as well as the hydrozoan family Stylasteridae (hydrocorals) [15]. Cold water corals do not need light to function. They obtain their nutrients and energy completely from trapping plankton and organic particles in passing currents. Lophelia pertusa has been shown to incorporate everything from dissolved and particular organic carbon to algal biomass to small zooplankton.
Although the majority of the species-level diversity is in the solitary corals, some of the CWC (e.g. Lophelia pertusa) form extensive reef structures, occasionally accumulating into large carbonate mounds, or bioherms (Cordes et al., 2016[4]). These reefs function as refuges, feeding areas and nurseries for many species, including commercially important fish[16]. CWC reefs are also linked to a high biodiversity.
All CWCs are extremely fragile and vulnerable to physical disturbance. The polyps cannot escape disturbances and their structures are extremely brittle. They also grow extremely slow (there are no zooxanthellae in the dark), so it takes a long time to rebuild the reef when it is damaged. A further disadvantage is that they cannot respond to fast-occurring changes in their environment, as they are adapted to stable conditions (WWF, 2001[17]). Trawl gear can damage CWC communities by reducing or changing coral abundances, diversity and community or the removal of structuring species.
Coral reef ecosystem
Coral reefs provide a structured habitat with a lot of niches for many species. They support a highly diverse community, comprising faunal biomass that is orders of magnitude above that of the surrounding area. They also serve as important spawning, nursery, breeding and feeding areas for a multitude of fishes and invertebrates and habitat for transient diel vertical migrators. This holds both for warm-water and cold-water reefs.
A diverse coral-associated fauna depends on the host coral on which they live as epibionts, either as surface encrusting epifauna or by living partially embedded in the coral skeleton. This symbiotic relationship can be either facultative, where the symbiont can survive without the host, or obligatory, where the associate cannot exist without the host or inversely (e.g. Zooxanthellae). Some epibionts provide protection from predators and disease or provide cleaning services, while others can wound their hosts (e.g., boring mussel Leiosolenus spp.), impede growth or completely suffocate the host. High densities of some symbiont taxa can be harmful to the host coral if skeletal integrity is compromised or if too many polyps are damaged. High densities of symbionts (e.g., coral barnacle Pyrgomatidae, Christmas tree worm Spirobranchus spp., Leiosolenus spp.) can occur under influence of eutrophication[18].
Many animals of the warm-water reefs are sessile or move very slowly. Sponges are found at deeper parts of the reef front. Smaller animals are found in the waters behind the reef. Anemones remain fixed in one place by a muscular, mucus-secreting basal disk. If the conditions become unfavorable, some sessile species can release themselves and crawl over the bottom, searching for a more suitable area. Fanworms or featherduster worms are represented by two families: the serpulid worms (Fig. 9a), which produce tubes of calcium carbonate, and sabellid worms, which form tubes of sand and small particles pasted together by mucus.
Organisms that can be found on the branches of coral reefs are: fish (Fig. 9b), crustaceans, starfish, molluscs, brittle stars (Fig. 9c), sea pens (Fig. 9d), sea urchins and squids. Sponges, bryozoans, hydroids, and some other coral species are found on the coral itself. The octopus (Fig. 9e) is one of the most formidable predators. It can rapidly change its color in response to its background (camouflage) and has very sharp eyesight. Octopuses have the ability to squeeze through small spaces where potential predators cannot gain access. Although the octopus is very well adapted to the coral reef environment, moray eels are the dominant nocturnal predators. They live in cracks, crevices and holes. At night, they come out to feed on shrimps, crabs, octopuses and other fish. During the day, barracudas (Fig. 9f) take their place as dominant predator (Karleskint, 1998[10]).
Fig. 9a. Serpulid worm (Serpula columbiana)
Fig. 9b. Moray eel
Fig. 9c. Brittle star
Fig. 9e. Common octopus (Octopus vulgaris) [19]
Fig. 9f. Barracuda (Sphyraena barracuda). Photo credit NOAA.
Threats
Natural causes are:
- Storms and tidal emersions;
- El Niño: change of sea surface temperatures outside the tolerance range of the local coral species
- Predation by fishes, marine worms, barnacles, crabs, snails and sea stars
- Dust outbreaks.
Human-induced causes are:
- Chemical pollution: see the article Threats to Coral Reefs: the Effects of Chemical Pollution.
- Eutrophication: Many eutrophicated regions which in the past were renowned for their corals are now dominated by macro algae and filamentous algae and expansion of seagrass beds (Bell, 1992[20]).
- Fishery: Coral is easily damaged by fishing gear, especially bottom trawls. Even worse are destructive fishing practices, such as cyanide and dynamite fishing, which are still prevalent in poorly regulated and enforced maritime areas in Southeast Asia.
- Dredging and dumping: Direct cutting through the reef and release of plumes of suspended sediment, which can settle on coral reefs and damage them from food and sunlight starvation.
- Coral mining: Extraction of coral for use as a building material.
Climate change impact on coral reefs
To date, the above human activities are the main cause of the widespread decline of coral reefs. An (even greater) looming threat to coral reefs is climate change, which is accompanied by increasing seawater temperature and increasing seawater acidity. Of all coastal ecosystems, the effects of climate change are globally the most threatening to coral reefs (Ani and Robson, 2021[21]). A strong relationship has been observed between short periods of elevated sea temperature, mass coral bleaching and mortality within warm-water coral reefs (Hoegh-Guldberg, 1999[22]). There is also strong experimental evidence that net ecosystem calcification and net community production will be altered when concentrations of dissolved CO2 in seawater increase (Noonan et al., 2018[23]; Comeau et al., 2019[24]; Doo et al., 2019[25]). A comprehensive review leads to the conclusion that coral reefs are unlikely to be able to adapt at rates sufficient to keep up with the rapid ocean warming (0.1–0.15 oC per decade) and acidification, especially given the fact that corals are long-lived and therefore have a slow evolution (Hoegh-Guldberg et al., 2017[26]). However, there is some evidence that colonization by recruits immigrating from warmer locations can help adapt to higher temperatures, assuming sufficiently slow warming and the absence of other stressors such as acidification and pollution (Matz et al., 2020[27]). Transplantation of bleaching-resistant coral species could also be an option (Barott et al., 2020[28]).
Coral reef restoration and creation
Coral reefs are very effective wave attenuators under storm conditions, besides offering an essential habitat for many marine organisms. Restoring degraded coral reefs can be an advantageous alternative to artificial coastal protection structures. Good results have been achieved with techniques involving coral gardening as intermediate step, where fragmented or recruited corals are grown in sheltered sites before transplantation to natural habitats[29]. New reefs can be created in a suitable environment with artificial materials (e.g. rock-filled steel baskets[30]) serving as support for colonization by corals. Extension of coral reefs has been experimented with success by transplanting corals on a substrate of artificial reefs[31].
Related articles
External link
References
- Jump up ↑ http://en.wikipedia.org/wiki/Coral_reef
- Jump up ↑ NOAA National Ocean Service Education: Corals
- Jump up ↑ Barnes R.D. 1987. Invertebrate Zoology. Fifth edition. Fort Worth, TX. Harcourt Brace Jovanovich College Publishers. 92-96, 127-134, 149-162
- ↑ Jump up to: 4.0 4.1 Cordes, E., Arnaud-Haond, S., Bergstad, O-A., Costa Falcao, A.P., Fereiwald, A., Toberts, J.M. and Bernal, P. 2016. The First Global Integrated Marine Assessment, World Ocean Assessment, Chapter 42. Cold-Water Corals. Regular Process for Global Reporting and Assessment of the State of the Marine Environment. UN
- Jump up ↑ De Leo, F. C., Smith, C. R., Rowden, A. A., Bowden, D. A. and Clark, M. R. 2010. Submarine canyons: hotspots of benthic biomass and productivity in the deep sea. Proc. R. Soc. B Biol. Sci. 277: 2783–2792
- Jump up ↑ Roberts, J.M., Wheeler, A.J., Freiwald, A. and Cairns, S.D. 2009. Cold-water Corals: The Biology and Geology of Deep-sea Coral Habitats. Cambridge: Cambridge University Press
- ↑ Jump up to: 7.0 7.1 Hebbeln, D., Wienberg, C., Dullo, W-C., Freiwald, A., Mienis, F., Orejas, C. and Titschack, J. 2020. Cold-water coral reefs thriving under hypoxia. Coral Reefs 39: 853–859
- Jump up ↑ Ta, K., Song, X., Wei, Z., Du, M., Xu, H., Chen, S., Li, J., Liu, S. and Peng, X. 2024. Oasis of the deep: Cold-water corals of the South China Sea. Marine Environmental Research 195: 106354
- Jump up ↑ UNEP/GRID-Arendal - Hugo Ahlenius
- ↑ Jump up to: 10.0 10.1 10.2 Karleskint G. 1998. Introduction to marine biology. Harcourt Brace College Publishers. p.378
- Jump up ↑ http://nl.wikipedia.org/wiki/Hersenkoraal - Photo credit Albert Kok
- Jump up ↑ http://en.wikipedia.org/wiki/Dendrogyra - Photo credit NOAA
- Jump up ↑ Kench, P.S. and Owen, S.D. 2022. Coral Systems. Ch. 8.22 of Treatise on Geomorphology (ed. Shroder, J.F.), Elsevier
- Jump up ↑ Roberts, J.M., Changing Oceans Expedition 2012, funded by UK Ocean Acidification programme (NERC, DECC, Defra)
- Jump up ↑ Cairns, S. D. 2007. Deep-water corals: an overview with special reference to diversity and distribution of deep-water scleractinian corals. Bull. Mar. Sci. 81: 311–322
- Jump up ↑ Roberts, J. M., Wheeler, A. J. and Freiwald, A. 200). Reefs of the deep: the biology and geology of cold-water coral ecosystems. Science 312: 543–547
- Jump up ↑ WWF. 2001. Cold water corals: fragile havens in the deep. p. 12
- Jump up ↑ van der Schoot, R.J. and Hoeksema, B.W. 2022. Abundance of coral-associated fauna in relation to depth and eutrophication along the leeward side of Curaçao, southern Caribbean. Marine Environmental Research 181, 105738
- Jump up ↑ http://en.wikipedia.org/wiki/Octopus - Photo credit Albert Kok
- Jump up ↑ Bell, P.R.F. 1992. Eutrophication and coral reefs—Some examples in the Great Barrier Reef Lagoon. Water Research 26: 553–568
- Jump up ↑ Ani, C.J. and Robson, B. 2021. Responses of marine ecosystems to climate change impacts and their treatment in biogeochemical ecosystem models. Marine Pollution Bulletin 166, 112223
- Jump up ↑ Hoegh-Guldberg, O. 1999. Coral bleaching, climate change and the future of the world’s coral reefs. Mar. Freshw. Res. 50, 839–866. doi: 10.1071/MF99078
- Jump up ↑ Noonan, S.H.C., Kluibenschedl, A. and Fabricius, K.E. 2018. Ocean acidification alters early successional coral reef communities and their rates of community metabolism. PLoS ONE 13(5): e0197130
- Jump up ↑ Comeau, S., Cornwall, C. E., DeCarlo, T. M., Doo, S. S., Carpenter R. C. and McCulloch, M. T. 2019. Resistance to ocean acidification in coral reef taxa is not gained by acclimatization. Nature Climate Change volume 9: 477–483
- Jump up ↑ Doo, S.S., Edmunds, P.J. and Carpenter, R.C. 2019. Ocean acidification effects on in situ coral reef metabolism. Nature Scientific Reports 9:12067 https://doi.org/10.1038/s41598-019-48407-7
- Jump up ↑ Hoegh-Guldberg, O., Poloczanska, E.S., Skirving, W. and Dove, S. 2017. Coral Reef Ecosystems under Climate Change and Ocean Acidification. Front. Mar. Sci. 4: 158. doi: 10.3389
- Jump up ↑ Matz, M.V., Treml, E.A. and Haller, B.C. 2020. Estimating the potential for coral adaptation to global warming across the Indo-Pacific. Glob. Change Biol. 26: 3473-3481
- Jump up ↑ Barott, K.L., Huffmyer, A.S., Davidson, J.M., Lenz, E.A., Matsuda, S.B., Hancock, J.R., Innis, T., Drury, C., Putnam, H.M. and Gates, R.D. 2021. Coral bleaching response is unaltered following acclimatization to reefs with distinct environmental conditions. PNAS 118, 22
- Jump up ↑ Young, C. N., Schopmeyer, S. A., and Lirman, D. 2012. A review of reef restoration and coral propagation using the threatened genus Acropora in the Caribbean and Western Atlantic. Bul. Mar. Sci. 88: 1075–1098
- Jump up ↑ Reguero, B.J., Beck, M.W., Agostini, V.N., Kramer, P. and Hancock, B. 2018. Coral reefs for coastal protection: A new methodological approach and engineering case study in Grenada. Journal of Environmental Management 210: 146-161
- Jump up ↑ Perkol-Finkel, S. and Benayahu, Y. 2009. The role of differential survival patterns in shaping coral communities on neighboring artificial and natural reefs. Journal of Experimental Marine Biology and Ecology 369: 1–7
Please note that others may also have edited the contents of this article.
|